Rimexolone
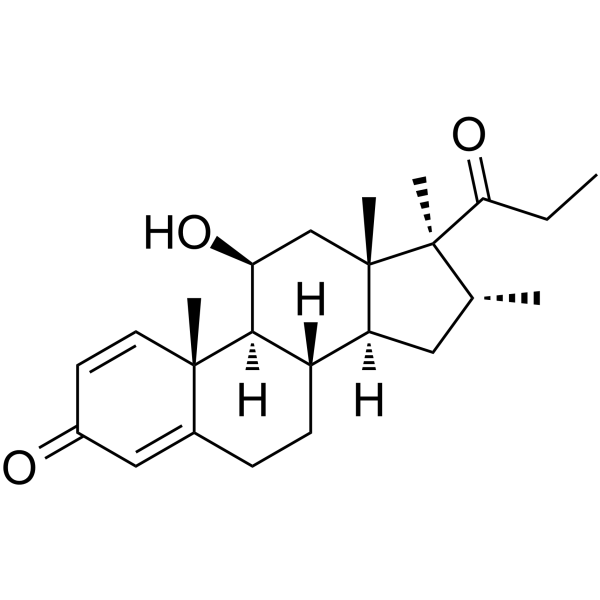
Rimexolone structure
|
Common Name | Rimexolone | ||
|---|---|---|---|---|
| CAS Number | 49697-38-3 | Molecular Weight | 370.525 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 507.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H34O3 | Melting Point | 258-268ºC | |
| MSDS | N/A | Flash Point | 274.5±26.6 °C | |
Use of RimexoloneRimexolone (Org 6216) is a glucocorticoid steroid with anti-inflammatory activity. Rimexolone can be used as a 1% ophthalmic suspension for the management of ocular inflammation[1][2]. |
| Name | Rimexolone |
|---|---|
| Synonym | More Synonyms |
| Description | Rimexolone (Org 6216) is a glucocorticoid steroid with anti-inflammatory activity. Rimexolone can be used as a 1% ophthalmic suspension for the management of ocular inflammation[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Rimexolone (Org 6216) shows highly enhanced persistence at the injection site, minimal systematic effects and virtually does not induce skin artrophy[1]. |
| In Vivo | Rimexolone (Org 6216) (0-450 μg/mouse; intraarticular injection; once) shows prolonged anti-inflammatory action in mice with monoarticular antigen-induced arthritis[1]. Animal Model: Male, 8-12 weeks old C57Black/6 mice with antigen induced arthritis[1] Dosage: 5, 25, 50, 150, 250, 450 μg/mouse Administration: Intraarticular injection, once Result: Suppressed the arthritis in a dose-dependent manner. Significantly prevented osteophyte formation at 450 and 150 μg. Resulted in a decrease of the inhibition of the chondrocyte proteoglycan synthesis. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 507.0±50.0 °C at 760 mmHg |
| Melting Point | 258-268ºC |
| Molecular Formula | C24H34O3 |
| Molecular Weight | 370.525 |
| Flash Point | 274.5±26.6 °C |
| Exact Mass | 370.250793 |
| PSA | 54.37000 |
| LogP | 4.01 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.559 |
|
~97% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
|
~% 
Rimexolone CAS#:49697-38-3 |
| Literature: Conrow, Raymond E. Journal of Organic Chemistry, 1999 , vol. 64, # 3 p. 1042 - 1044 |
| Rimexolone |
| (11β,16α,17β)-11-Hydroxy-16,17-dimethyl-17-(1-oxopropyl)androsta-1,4-dien-3-one |
| 11β-Hydroxy-16α,17α-dimethyl-17-propionylandrosta-1,4-dien-3-one |
| 11b-Hydroxy-16a,17a-dimethyl-17-propionylandrosta-1,4-dien-3-one |
| (8S,9S,10R,11S,13S,14S,16R,17S)-11-Hydroxy-10,13,16,17-tetramethyl-17-propionyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one |
| (8S,9S,10R,11S,13S,14S,16R,17S)-11-Hydroxy-10,13,16,17-tetramethyl-17-propanoyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-on |
| (8S,9S,10R,11S,13S,14S,16R,17S)-11-hydroxy-10,13,16,17-tetramethyl-17-propanoyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one |
| (11b,16a,17b)-11-Hydroxy-16,17-dimethyl-17-(1-oxopropyl)androsta-1,4-dien-3-one |
| Asoprisnil ecamate |
| Androsta-1,4-dien-3-one, 11-hydroxy-16,17-dimethyl-17-(1-oxopropyl)-, (11β,16α,17β)- |
| (8S,9S,10R,11S,13S,14S,16R,17S)-11-hydroxy-10,13,16,17-tétraméthyl-17-propanoyl-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrén-3-one |
| 11β-Hydroxy-16α,17,21-trimethyl-1,4-pregnadien-3,20-dion |
| (8S,9S,10R,11S,13S,14S,16R,17S)-11-hydroxy-10,13,16,17-tetramethyl-17-propanoyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one (non-preferred name) |
| (8S,9S,10R,13S,14S,16R,17S)-11-Hydroxy-10,13,16,17-tetramethyl-17-propionyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one (non-preferred name) |
| VEXOL |

![(8S,9S,10S,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-iodoacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one structure](https://image.chemsrc.com/caspic/354/49757-06-4.png)