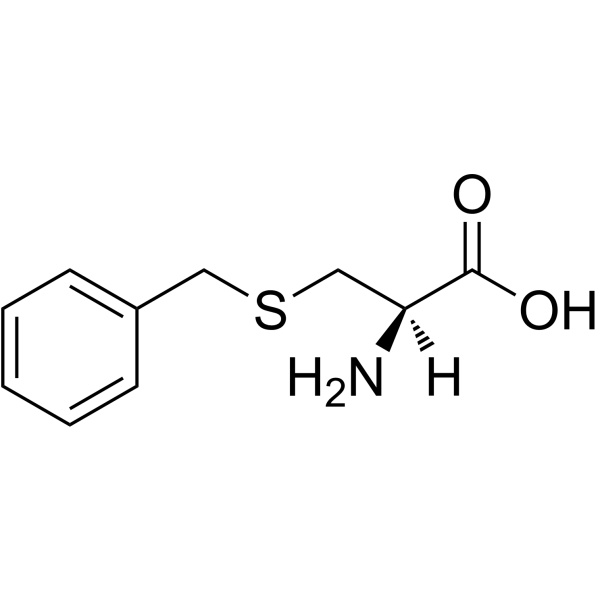
Boc-Cys(Bzl)-OH

Boc-Cys(Bzl)-OH structure
|
Common Name | Boc-Cys(Bzl)-OH | ||
|---|---|---|---|---|
| CAS Number | 5068-28-0 | Molecular Weight | 311.397 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 481.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C15H21NO4S | Melting Point | 86-88 °C | |
| MSDS | Chinese USA | Flash Point | 244.8±28.7 °C | |
Use of Boc-Cys(Bzl)-OH(R)-3-(Benzylthio)-2-((tert-butoxycarbonyl)amino)propanoic acid is a cysteine derivative[1]. |
| Name | Boc-S-Benzyl-L-cysteine |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-3-(Benzylthio)-2-((tert-butoxycarbonyl)amino)propanoic acid is a cysteine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.2±45.0 °C at 760 mmHg |
| Melting Point | 86-88 °C |
| Molecular Formula | C15H21NO4S |
| Molecular Weight | 311.397 |
| Flash Point | 244.8±28.7 °C |
| Exact Mass | 311.119141 |
| PSA | 100.93000 |
| LogP | 3.93 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.554 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T+ |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2930909090 |
|
~99% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Koerber-Ple; Massiot Journal of Heterocyclic Chemistry, 1995 , vol. 32, # 4 p. 1309 - 1315 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Koerber-Ple; Massiot Journal of Heterocyclic Chemistry, 1995 , vol. 32, # 4 p. 1309 - 1315 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Anderson; McGregor Journal of the American Chemical Society, 1957 , vol. 79, p. 6180,6181 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: AXYS PHARMACEUTICALS, INC. Patent: WO2004/838 A1, 2003 ; Location in patent: Page 85 ; WO 2004/000838 A1 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Richter, Lutz S.; Marsters Jr., James C.; Gadek, Thomas R. Tetrahedron Letters, 1994 , vol. 35, # 11 p. 1631 - 1634 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Khan,S.A.; Sivanandaiah,K.M. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1977 , vol. 15, p. 80 - 81 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Itoh,M. et al. Bulletin of the Chemical Society of Japan, 1977 , vol. 50, p. 718 - 721 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Broadbent,W. et al. Journal of the Chemical Society [Section] C: Organic, 1967 , p. 2632 - 2636 |
|
~% 
Boc-Cys(Bzl)-OH CAS#:5068-28-0 |
| Literature: Ageeva; Kurzeev; Kazankov Russian Journal of Organic Chemistry, 2007 , vol. 43, # 4 p. 548 - 552 |
| Precursor 10 | |
|---|---|
| DownStream 6 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Formation of a dehydroalanyl residue from S-benzylcysteine upon HF cleavage of a [Sar1, Cys8]-angiotensin II peptide resin.
Int. J. Pept. Protein Res. 38(6) , 601-2, (1991)
|
| Boc-Cys(Bzl)-OH |
| N-Boc-S-Benzyl-L-Cys-OH |
| N-Boc-S-benzyl-L-cysteine |
| L-Cysteine, N-((1,1-dimethylethoxy)carbonyl)-S-(phenylmethyl)- |
| Boc-S-Benzyl-L-Cysteine |
| N-tert-butoxycarbonyl-S-benzylcysteine |
| N-α-t-Boc-S-benzyl-L-cysteine |
| N-(tert-Butoxycarbonyl)-S-benzyl-L-cysteine |
| BOC-S-BENZYL-CYSTEINE |
| (R)-3-(Benzylthio)-2-((tert-butoxycarbonyl)amino)propanoic acid |
| t-butyloxycarbonyl-S-benzyl-L-cysteine |
| EINECS 225-772-8 |
| MFCD00065567 |
| S-Benzyl-N-(tert-butoxycarbonyl)-L-cysteine |
| S-benzyl N-Boc-cysteine |
| S-Benzyl-N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-cysteine |
| L-Cysteine, N-[(1,1-dimethylethoxy)carbonyl]-S-(phenylmethyl)- |

![L-Cysteine,N-[(1,1-dimethylethoxy)carbonyl]-S-(phenylmethyl)-, 4-nitrophenyl ester structure](https://image.chemsrc.com/caspic/120/3560-17-6.png)
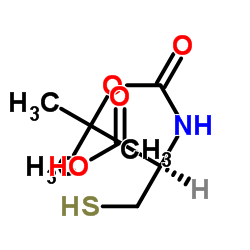 CAS#:20887-95-0
CAS#:20887-95-0 CAS#:10389-65-8
CAS#:10389-65-8 CAS#:3401-33-0
CAS#:3401-33-0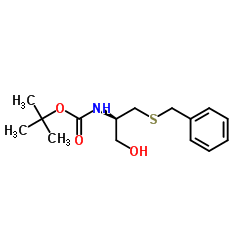 CAS#:139428-96-9
CAS#:139428-96-9![3-benzyloxy-estra-1,3,5(10),16-tetraeno[17,16-e]-2'-(S-benzyl-N-tert-butoxycarbonyl-L-cysteinylamino)pyrimidine structure](https://image.chemsrc.com/caspic/311/669056-34-2.png) CAS#:669056-34-2
CAS#:669056-34-2
