DL-Mevalonolactone
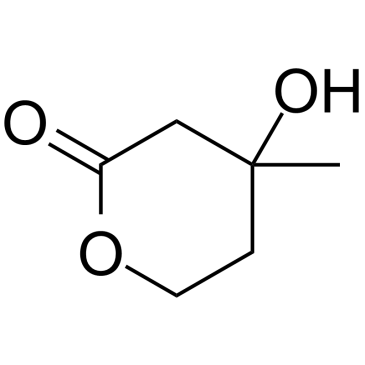
DL-Mevalonolactone structure
|
Common Name | DL-Mevalonolactone | ||
|---|---|---|---|---|
| CAS Number | 674-26-0 | Molecular Weight | 130.14200 | |
| Density | 1.189 g/cm3 | Boiling Point | 145-150 °C5 mm Hg(lit.) | |
| Molecular Formula | C6H10O3 | Melting Point | 28 °C(lit.) | |
| MSDS | USA | Flash Point | >230 °F | |
Use of DL-MevalonolactoneDL-Mevalonolactone ((±)-Mevalonolactone;Mevalolactone) is the δ-lactone form of mevalonic acid, a precursor in the mevalonate pathway. DL-Mevalonolactone (Mevalonolactone) decreases mitochondrial membrane potential (∆Ψm), NAD(P)H content and the capacity to retain Ca2+ in the brain, besides inducing mitochondrial swelling[1][2]. |
| Name | DL-Mevalonolactone |
|---|---|
| Synonym | More Synonyms |
| Description | DL-Mevalonolactone ((±)-Mevalonolactone;Mevalolactone) is the δ-lactone form of mevalonic acid, a precursor in the mevalonate pathway. DL-Mevalonolactone (Mevalonolactone) decreases mitochondrial membrane potential (∆Ψm), NAD(P)H content and the capacity to retain Ca2+ in the brain, besides inducing mitochondrial swelling[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.189 g/cm3 |
|---|---|
| Boiling Point | 145-150 °C5 mm Hg(lit.) |
| Melting Point | 28 °C(lit.) |
| Molecular Formula | C6H10O3 |
| Molecular Weight | 130.14200 |
| Flash Point | >230 °F |
| Exact Mass | 130.06300 |
| PSA | 46.53000 |
| LogP | 0.07440 |
| Index of Refraction | n20/D 1.473(lit.) |
| InChIKey | JYVXNLLUYHCIIH-UHFFFAOYSA-N |
| SMILES | CC1(O)CCOC(=O)C1 |
| Storage condition | 2-8°C |
| Stability | Hygroscopic |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932999099 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells.
Br. J. Cancer 111(8) , 1562-71, (2014) The increasing usage of statins (the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) has revealed a number of unexpected beneficial effects, including a reduction in cancer risk.We investi... |
|
|
Synthesis and HMG-CoA reductase inhibition of 2-cyclopropyl-4-thiophenyl-quinoline mevalonolactones.
Bioorg. Med. Chem. 17(23) , 7915-23, (2009) A novel series of 2-cyclopropyl-4-thiophenyl quinoline-based mevalonolactones were synthesized from the substituted anilines by several reactions. Among them, (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-... |
|
|
Highly sensitive assay of HMG-CoA reductase activity by LC-ESI-MS/MS.
J. Lipid Res. 48(5) , 1212-20, (2007) We have developed a new sensitive and specific nonradioisotope assay method to measure the activity of HMG-CoA reductase, the rate-controlling enzyme in the cholesterol biosynthetic pathway. This meth... |
| (3RS)-mevalonolactone |
| MEVALONIC LACTONE |
| (+/-)-MEVALONOLACTONE |
| EINECS 211-615-0 |
| DL-MEVALOLACTONE |
| MFCD00006648 |
| DL-mevalonic acid lactone |
| D,L-MEVELONIC ACID LACTONE |
| MEVALONIC ACID LACTONE,DL |
| (RS)-mevalonolactone |
| (S/R)-mevalonolactone |
 CAS#:7564-64-9
CAS#:7564-64-9![5-[(4-nitrophenyl)methylcarbamoyloxy]-3-hydroxy-3-methylpentanoic acid Structure](https://image.chemsrc.com/caspic/387/246237-03-6.png) CAS#:246237-03-6
CAS#:246237-03-6 CAS#:34695-32-4
CAS#:34695-32-4 CAS#:114580-75-5
CAS#:114580-75-5 CAS#:246236-98-6
CAS#:246236-98-6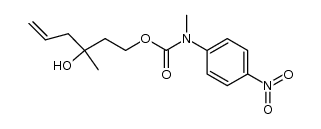 CAS#:246237-00-3
CAS#:246237-00-3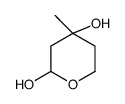 CAS#:99720-12-4
CAS#:99720-12-4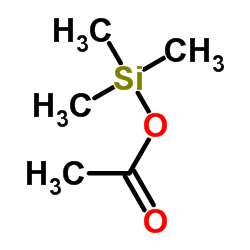 CAS#:2754-27-0
CAS#:2754-27-0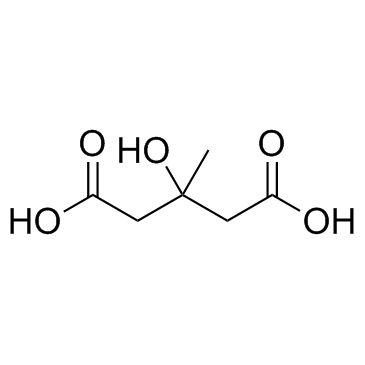 CAS#:503-49-1
CAS#:503-49-1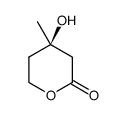 CAS#:19115-49-2
CAS#:19115-49-2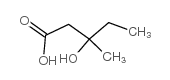 CAS#:150-96-9
CAS#:150-96-9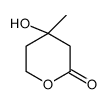 CAS#:19022-60-7
CAS#:19022-60-7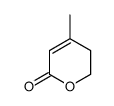 CAS#:2381-87-5
CAS#:2381-87-5 CAS#:5788-94-3
CAS#:5788-94-3 CAS#:5119-48-2
CAS#:5119-48-2