Echinocystic acid
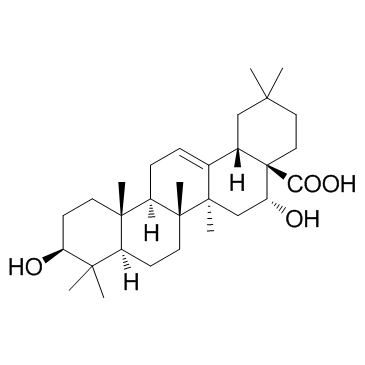
Echinocystic acid structure
|
Common Name | Echinocystic acid | ||
|---|---|---|---|---|
| CAS Number | 510-30-5 | Molecular Weight | 472.700 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 585.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C30H48O4 | Melting Point | 299-300ºC | |
| MSDS | N/A | Flash Point | 321.6±26.6 °C | |
Use of Echinocystic acidEchinocystic acid a pentacyclic triterpene isolated from the fruits of Gleditsia sinensis Lam, has potent antioxidant, anti-inflammatory and anti-tumor properties. In vitro: Echinocystic acid (EA) inhibit the formation of osteoclast. EA inhibit RANKL-induced NF-κB activation and ERK phosphorylation in BMMs. [1] EA inhibit IL-1β-induced inflammation in chondrocytes. [2]In vivo: Echinocystic acid reduces reserpine-induced pain/depression dyad in mice. [3] |
| Name | echinocystic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Echinocystic acid a pentacyclic triterpene isolated from the fruits of Gleditsia sinensis Lam, has potent antioxidant, anti-inflammatory and anti-tumor properties. In vitro: Echinocystic acid (EA) inhibit the formation of osteoclast. EA inhibit RANKL-induced NF-κB activation and ERK phosphorylation in BMMs. [1] EA inhibit IL-1β-induced inflammation in chondrocytes. [2]In vivo: Echinocystic acid reduces reserpine-induced pain/depression dyad in mice. [3] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 585.0±50.0 °C at 760 mmHg |
| Melting Point | 299-300ºC |
| Molecular Formula | C30H48O4 |
| Molecular Weight | 472.700 |
| Flash Point | 321.6±26.6 °C |
| Exact Mass | 472.355255 |
| PSA | 77.76000 |
| LogP | 7.58 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.568 |
| InChIKey | YKOPWPOFWMYZJZ-HJENMKQUSA-N |
| SMILES | CC1(C)CCC2(C(=O)O)C(O)CC3(C)C(=CCC4C5(C)CCC(O)C(C)(C)C5CCC43C)C2C1 |
| Storage condition | 2-8C |
|
~% 
Echinocystic acid CAS#:510-30-5 |
| Literature: Miyase, Toshio; Kohsaka, Hiromi; Ueno, Akira Phytochemistry (Elsevier), 1992 , vol. 31, # 6 p. 2087 - 2091 |
|
~% 
Echinocystic acid CAS#:510-30-5 |
| Literature: Miyase, Toshio; Kohsaka, Hiromi; Ueno, Akira Phytochemistry (Elsevier), 1992 , vol. 31, # 6 p. 2087 - 2091 |
|
~%
Detail
|
| Literature: Konoshima, Takao; Fukushima, Hiroyuki; Inui,Hideo; Sato, Keiko; Sawada, Tokunosuke Phytochemistry (Elsevier), 1981 , vol. 20, p. 139 - 142 |
|
~% 
Echinocystic acid CAS#:510-30-5 |
| Literature: Kuang, Hai-Xue; Wang, Zhi-Bin; Wang, Qiu-Hong; Yang, Bing-You; Xiao, Hong-Bin; Okada, Yoshihito; Okuyama, Tohru Chemistry and Biodiversity, 2013 , vol. 10, # 4 p. 703 - 710 |
| (4aR,5R,6aS,6bR,8aR,10S,12aR,12bR,14bS)-5,10-Dihydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydro-4a(2H)-picenecarboxylic acid |
| Echicystic acid |
| (3β,16α)-3,16-Dihydroxyolean-12-en-28-oic acid |
| Albizziagenin |
| Echinocystsaeure |
| 3-B,16-A-DIHYDROXYOLEAN-12-EN-28-OIC ACID |
| echinaystic acid |
| Olean-12-en-28-oic acid, 3,16-dihydroxy-, (3β,16α)- |
| Echinocystic |

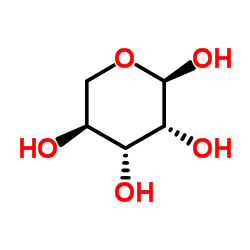
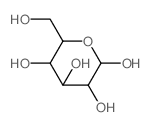
![1-O-[(3β,16α)-3-{[β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl]oxy}-16-hydroxy-28-oxoolean-12-en-28-yl]-β-D-glucopyranose structure](https://image.chemsrc.com/caspic/060/1431884-70-6.png)