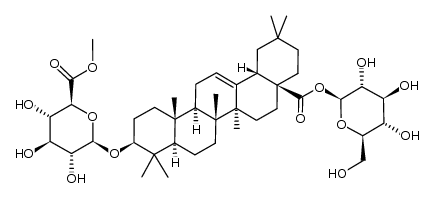
Chikusetsusaponin IVa
Modify Date: 2025-08-21 13:47:17
Chikusetsusaponin IVa structure
|
Common Name | Chikusetsusaponin IVa | ||
|---|---|---|---|---|
| CAS Number | 51415-02-2 | Molecular Weight | 794.965 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 873.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C42H66O14 | Melting Point | 218-220 ºC (methanol , water ) | |
| MSDS | N/A | Flash Point | 255.6±27.8 °C | |
Use of Chikusetsusaponin IVaChikusetsusaponin IVa a major active ingredient of triterpenoid saponins, exerts antithrombotic effects, including minor hemorrhagic events. This appears to be important for the development of new therapeutic agents. a novel AMPK activator that is capable of bypassing defective insulin signalling and could be useful for the treatment of T2DM or other metabolic disorders.IC50 Value: 199.4 ± 9.1 μM (inhibiting thrombin-induced fibrinogen clotting) Target: In vitro: Using biochemical and pharmacological methods, it proves that chikusetsusaponin IVa prolongs the recalcification time, prothrombin time, activated partial thromboplastin time, and thrombin time of normal human plasma in a dose-dependent manner; inhibits the amidolytic activity of thrombin and factor Xa upon synthetic substrates S2238 and S2222; inhibits thrombin-induced fibrinogen clotting (50% inhibition concentration, 199.4 ± 9.1 μM); inhibits thrombin- and collagen-induced platelet aggregation. Chikusetsusaponin IVa can also preferentially inhibits thrombin in a competitive manner (K(i)=219.6 μM) [1]. Chikusetsusaponin IVa suppresses the production of iNOS, COX-2, IL-1β, IL-6, and TNF-α in LPS-stimulated THP-1 cells likely by inhibiting NF-κB activation and ERK, JNK, and p38 signal pathway phosphorylation [2].In vivo: Studies were performed on type 2 diabetic mellitus (T2DM) rats given CHS for 28 days to test the antihyperglycemic activity. Oral administration of CHS dose-dependently increased the level of serum insulin and decreased the rise in blood glucose level [3]. |
| Name | chikusetsusaponin-IVa |
|---|---|
| Synonym | More Synonyms |
| Description | Chikusetsusaponin IVa a major active ingredient of triterpenoid saponins, exerts antithrombotic effects, including minor hemorrhagic events. This appears to be important for the development of new therapeutic agents. a novel AMPK activator that is capable of bypassing defective insulin signalling and could be useful for the treatment of T2DM or other metabolic disorders.IC50 Value: 199.4 ± 9.1 μM (inhibiting thrombin-induced fibrinogen clotting) Target: In vitro: Using biochemical and pharmacological methods, it proves that chikusetsusaponin IVa prolongs the recalcification time, prothrombin time, activated partial thromboplastin time, and thrombin time of normal human plasma in a dose-dependent manner; inhibits the amidolytic activity of thrombin and factor Xa upon synthetic substrates S2238 and S2222; inhibits thrombin-induced fibrinogen clotting (50% inhibition concentration, 199.4 ± 9.1 μM); inhibits thrombin- and collagen-induced platelet aggregation. Chikusetsusaponin IVa can also preferentially inhibits thrombin in a competitive manner (K(i)=219.6 μM) [1]. Chikusetsusaponin IVa suppresses the production of iNOS, COX-2, IL-1β, IL-6, and TNF-α in LPS-stimulated THP-1 cells likely by inhibiting NF-κB activation and ERK, JNK, and p38 signal pathway phosphorylation [2].In vivo: Studies were performed on type 2 diabetic mellitus (T2DM) rats given CHS for 28 days to test the antihyperglycemic activity. Oral administration of CHS dose-dependently increased the level of serum insulin and decreased the rise in blood glucose level [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 873.3±65.0 °C at 760 mmHg |
| Melting Point | 218-220 ºC (methanol , water ) |
| Molecular Formula | C42H66O14 |
| Molecular Weight | 794.965 |
| Flash Point | 255.6±27.8 °C |
| Exact Mass | 794.445251 |
| PSA | 232.90000 |
| LogP | 6.37 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.611 |
| InChIKey | YOSRLTNUOCHBEA-SGVKAIFKSA-N |
| SMILES | CC1(C)CCC2(C(=O)OC3OC(CO)C(O)C(O)C3O)CCC3(C)C(=CCC4C5(C)CCC(OC6OC(C(=O)O)C(O)C(O)C6O)C(C)(C)C5CCC43C)C2C1 |
| Safety Phrases | 24/25 |
|---|
|
~% 
Chikusetsusapon... CAS#:51415-02-2 |
| Literature: Davidyants, E. S.; Putieva, Zh. M.; Bandyukova, V. A.; Abubakirov, N. K. Chemistry of Natural Compounds, 1986 , vol. 22, # 1 p. 58 - 60 Khimiya Prirodnykh Soedinenii, 1986 , vol. 22, # 1 p. 63 - 66 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| Chikusetsusaponin |
| Chikusetsusaponin Iva |
| 1-O-[(3α,5ξ,9ξ)-3-(β-D-Glucopyranuronosyloxy)-28-oxoolean-12-en-28-yl]-β-D-glucopyranose |
| GLUCOPYRANOSIDURONIC ACID |
| Silphioside G |
| Chikusetsu saponin Ⅳa |
| β-D-Glucopyranose, 1-O-[(3α,5ξ,9ξ)-3-(β-D-glucopyranuronosyloxy)-28-oxoolean-12-en-28-yl]- |
| β-D-Glucopyranose, 1-O-[(3β)-3-(β-D-glucopyranuronosyloxy)-28-oxoolean-12-en-28-yl]- |
| Chikusetsu Saponin IVa |
| 1-O-[(3β)-3-(β-D-Glucopyranuronosyloxy)-28-oxoolean-12-en-28-yl]-β-D-glucopyranose |
| Calenduloside F |
| Chikusetsusaponin IV |