Cimetidine

Cimetidine structure
|
Common Name | Cimetidine | ||
|---|---|---|---|---|
| CAS Number | 51481-61-9 | Molecular Weight | 252.339 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 476.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C10H16N6S | Melting Point | 139-144°C | |
| MSDS | Chinese USA | Flash Point | 241.8±31.5 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of CimetidineCimetidine is a histamine-2 (H2) receptor antagonist.IC50 Value: Target: Histamine-2 Receptorin vitro: Cimetidine, a partial agonist for H2R, has a pharmacological profile different from ranitidine and famotidine, possibly contributing to its antitumor activity on gastrointestinal cancers [1]. Cimetidine had no effect on the uptake and cytotoxicity of cisplatin in ovarian cancer cells with high OCT2 mRNA levels (IGROV-1 cells) [2]. Cimetidine showed no effect on proliferation, survival, migration and invasion of 3LL cells. Cimetidine reversed MDSC-mediated T-cell suppression, and improved IFN-γ production. [3]. Cimetidine-mediated down-regulation of NCAM involved suppression of the nuclear translocation of NF-kappaB, a transcriptional activator of NCAM gene expression [4].in vivo: the antitumor efficacy of cisplatin in mice bearing luciferase-tagged IGROV-1 xenografts was unaffected by cimetidine (P = 0.39). Data obtained in 18 patients receiving cisplatin (100 mg/m(2)) in a randomized crossover fashion with or without cimetidine (800 mg × 2) revealed that cimetidine did not alter exposure to unbound cisplatin [2]. cimetidine reduced CD11b(+)Gr-1(+) myeloid derived-suppressive cell (MDSC) accumulation in spleen, blood and tumor tissue of tumor-bearing mice [3]. Cimetidine exerts a beneficial effect on periodontal disease in rats, decreasing the RANKL/OPG ratio in gingival connective tissue and reducing alveolar bone resorption [5]. |
| Name | cimetidine |
|---|---|
| Synonym | More Synonyms |
| Description | Cimetidine is a histamine-2 (H2) receptor antagonist.IC50 Value: Target: Histamine-2 Receptorin vitro: Cimetidine, a partial agonist for H2R, has a pharmacological profile different from ranitidine and famotidine, possibly contributing to its antitumor activity on gastrointestinal cancers [1]. Cimetidine had no effect on the uptake and cytotoxicity of cisplatin in ovarian cancer cells with high OCT2 mRNA levels (IGROV-1 cells) [2]. Cimetidine showed no effect on proliferation, survival, migration and invasion of 3LL cells. Cimetidine reversed MDSC-mediated T-cell suppression, and improved IFN-γ production. [3]. Cimetidine-mediated down-regulation of NCAM involved suppression of the nuclear translocation of NF-kappaB, a transcriptional activator of NCAM gene expression [4].in vivo: the antitumor efficacy of cisplatin in mice bearing luciferase-tagged IGROV-1 xenografts was unaffected by cimetidine (P = 0.39). Data obtained in 18 patients receiving cisplatin (100 mg/m(2)) in a randomized crossover fashion with or without cimetidine (800 mg × 2) revealed that cimetidine did not alter exposure to unbound cisplatin [2]. cimetidine reduced CD11b(+)Gr-1(+) myeloid derived-suppressive cell (MDSC) accumulation in spleen, blood and tumor tissue of tumor-bearing mice [3]. Cimetidine exerts a beneficial effect on periodontal disease in rats, decreasing the RANKL/OPG ratio in gingival connective tissue and reducing alveolar bone resorption [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 476.2±55.0 °C at 760 mmHg |
| Melting Point | 139-144°C |
| Molecular Formula | C10H16N6S |
| Molecular Weight | 252.339 |
| Flash Point | 241.8±31.5 °C |
| Exact Mass | 252.115707 |
| PSA | 114.19000 |
| LogP | 0.07 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.632 |
| InChIKey | AQIXAKUUQRKLND-UHFFFAOYSA-N |
| SMILES | CN=C(NC#N)NCCSCc1nc[nH]c1C |
| Storage condition | 2-8°C |
| Water Solubility | 0.5 g/100 mL at 20 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R60 |
| Safety Phrases | S53-S26-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MF0035500 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Diclofenac toxicity in human intestine ex vivo is not related to the formation of intestinal metabolites.
Arch. Toxicol. 89(1) , 107-19, (2015) The use of diclofenac (DCF), a nonsteroidal anti-inflammatory drug, is associated with a high prevalence of gastrointestinal side effects. In vivo studies in rodents suggested that reactive metabolite... |
|
|
cIEF for rapid pKa determination of small molecules: a proof of concept.
Eur. J. Pharm. Sci. 63 , 14-21, (2014) A capillary isoelectric focusing (cIEF) method was developed for the determination of the ionization constants (pKa) of small molecules. Two approaches used to decrease the electroosmotic flow (EOF) w... |
|
|
Involvement of the H1 Histamine Receptor, p38 MAP Kinase, Myosin Light Chains Kinase, and Rho/ROCK in Histamine-Induced Endothelial Barrier Dysfunction.
Microcirculation 22 , 237-48, (2015) The mechanisms by which histamine increases microvascular permeability remain poorly understood. We tested the hypothesis that H1 receptor activation disrupts the endothelial barrier and investigated ... |
| 1-Cyano-2-methyl-3-(2-{[(4-methyl-1H-imidazol-5-yl)methyl]sulfanyl}ethyl)guanidine |
| Acinil |
| EINECS 257-232-2 |
| Cimetidine |
| Guanidine, N-cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)- |
| ULCIMET |
| 2-Cyano-1-methyl-3-[2-(5-methyl-1H-imidazol-4-yl-methylthio)ethyl]guanidine |
| 1-Cyano-3-methyl-2-(2-{[(4-methyl-1H-imidazol-5-yl)methyl]sulfanyl}ethyl)guanidine |
| Cimal |
| Guanidine, N-cyano-N''-methyl-N'-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl]- |
| N-Cyano-N'-methyl-N''-[2-[[(4-methyl-1H-imidazol-5-yl)methyl]thio]ethyl]guanidine |
| guanidine, N-cyano-N'-methyl-N''-[2-[[(4-methyl-1H-imidazol-5-yl)methyl]thio]ethyl]- |
| Dyspamet |
| Brumetadina |
| MFCD00133296 |
| Tagamet |
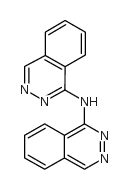 CAS#:103429-70-5
CAS#:103429-70-5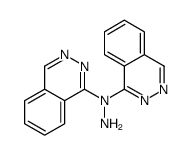 CAS#:103429-71-6
CAS#:103429-71-6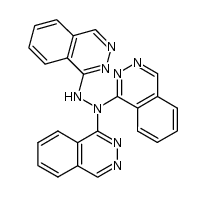 CAS#:103429-73-8
CAS#:103429-73-8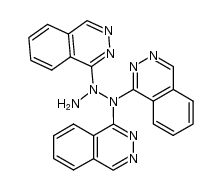 CAS#:103429-72-7
CAS#:103429-72-7 CAS#:26787-78-0
CAS#:26787-78-0
