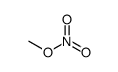
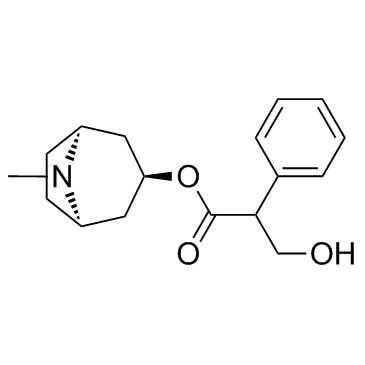
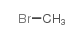atropine methyl nitrate
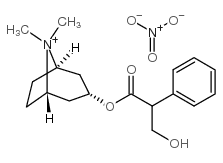
atropine methyl nitrate structure
|
Common Name | atropine methyl nitrate | ||
|---|---|---|---|---|
| CAS Number | 52-88-0 | Molecular Weight | 366.40900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C18H26N2O6 | Melting Point | 163°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
| Name | (8,8-dimethyl-8-azoniabicyclo[3.2.1]octan-3-yl) 3-hydroxy-2-phenylpropanoate,nitrate |
|---|---|
| Synonym | More Synonyms |
| Melting Point | 163°C |
|---|---|
| Molecular Formula | C18H26N2O6 |
| Molecular Weight | 366.40900 |
| Exact Mass | 366.17900 |
| PSA | 115.41000 |
| LogP | 2.31840 |
| Appearance of Characters | powder | white |
| Storage condition | ?20°C |
| Water Solubility | H2O: 50 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 + H330 |
| Precautionary Statements | P260-P264-P284-P301 + P310-P310 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| Safety Phrases | S22-S36 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | CK2800000 |
|
~% 
atropine methyl... CAS#:52-88-0 |
| Literature: Bayer and Co. Patent: DE138443 ; Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 7, p. 691 |
|
~% 
atropine methyl... CAS#:52-88-0 |
| Literature: Merck Patent: DE145996 ; Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 7, p. 692 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Validation of a rapid method of analysis using ultrahigh-performance liquid chromatography - tandem mass spectrometry for nitrogen-rich adulterants in nutritional food ingredients.
J. Chromatogr. A. 1373 , 106-13, (2014) A method for the rapid quantification of 9 potential nitrogen-rich economic adulterants (dicyandiamide, urea, biuret, cyromazine, amidinourea, ammeline, amidinourea, melamine, and cyanuric acid) in fi... |
|
|
Interleukin-18 expression increases in response to neurovascular damage following soman-induced status epilepticus in rats.
J. Inflamm. (Lond.) 12 , 43, (2015) Status epilepticus (SE) can cause neuronal cell death and impaired behavioral function. Acute exposure to potent acetylcholinesterase inhibitors such as soman (GD) can cause prolonged SE activity, mic... |
|
|
Core-shell magnetic molecularly imprinted polymers as sorbent for sulfonylurea herbicide residues.
J. Agric. Food Chem. 63(14) , 3634-45, (2015) Sulfonylurea herbicides are widely used at lower dosage for controlling broad-leaf weeds and some grasses in cereals and economic crops. It is important to develop a highly efficient and selective pre... |
| Pylostropin |
| Europen |
| Eumidrina |
| Eumydrine |
| Harvatrate |
| N-Methylatropine nitrate |
| EINECS 200-156-1 |
| Eumydrin |
| Ekomine |
| 8,8-dimethyl-3endo-DL-tropoyloxy-nortropanium,nitrate |
| Methylatropine nitrate |
| Atropine methonitrate |
| MFCD00050294 |
| 8,8-Dimethyl-3endo-DL-tropoyloxy-nortropanium,Nitrat |
| Metanite |
| Atropine methyl nitrate |