| Description |
Hesperetin is a natural flavanone, and acts as a potent and broad-spectrum inhibitor against human UGT activity.
|
| Related Catalog |
|
| In Vitro |
Hesperetin has the retention of antioxidant potential in self nano-emulsifying drug delivery system[1]. Hesperetin and NGR display broad-spectrum inhibition against human UGTs. Besides, Hesperetin exhibits strong inhibitory effects on UGT1A1, 1A3 and 1A9 (both IC50 and Ki values lower than 10 µM) and moderately inhibits UGT1A4, UGT1A7, UGT1A8 (IC50 values 29.68-63.87 µM)[2]. Hesperetin interacts with different types of proteins involving hydrogen bonds, pi-pi effects, pi-cation bonding and pi-sigma interactions with varying binding energies. Hesperetin exhibits drug-like properties which projects its potential as a therapeutic option for CHIKV infection[3]. Hesperetin dose-dependently reduces GCDCA-induced caspase-3 activity in cultured primary rat hepatocytes. Hesperetin also dose-dependently reduces CM-induced Nos2 (iNOS) expression in hepatocytes. Interestingly, hesperetin-induced expression of the antioxidant gene haem oxygenase 1 (HO-1) about fourfold compared with cytokine mixture alone[5].
|
| In Vivo |
Preadministration of Hesperetin (40 mg/kg b.w., oral) significantly attenuates the Cd-induced oxidative stress and mitochondrial dysfunction, restores the antioxidant and membrane-bound enzyme activities and decreases apoptosis in the brain of rats[4]. Hesperetin (200 mg/kg) attenuates Con A-induced hepatocyte apoptosis and hepatic Nos2 (iNOS) expression in mice. Hesperetin co-treatment also decreases the occurrence of apoptotic bodies, hydropic degeneration, nuclear fragments, autolysis and haemorrhage. The number of leukocytes infiltrated in liver tissue of mice with D-GalN/LPS-induced fulminant hepatitis are significantly decreased by hesperetin in a murine model[5].
|
| Kinase Assay |
First, 0.5 mL tissue homogenate is diluted with 1 mL water. Then, to this mixture, 2.5 mL ethanol and 1.5 mL chloroform (all reagents chilled) are added and shaken for 1 min at 4°C, then centrifuged. The enzyme activity in the supernatant is determined. The assay mixture contained 1.2 mL sodium pyrophosphate buffer (0.025 M, pH 8.3), 0.1 mL 186 mM phenazine methosulfate (PMS), 0.3 mL 30 mM Nitroblue tetrazolium (NBT), and 0.2 mL of nicotinamide adenine dinucleotide (NADH), and appropriately diluted enzyme preparation and water in a total volume of 3 mL. Reaction is initiated by the addition of NADH. After incubation at 30°C for 90 min, the reaction is stopped by the addition of 1 mL glacial acetic acid. The reaction mixture is stirred vigorously and shaken with 4 mL n-butanol. The intensity of the chromogen in the butanol layer is measured at 560 nm against a butanol blank. A system without enzyme served as control. One unit of enzyme activity is defined as 50% inhibition of NBT reduction in 1 min under the assay conditions.
|
| Animal Admin |
After 7 days of adjusting, the animals are randomly divided into 10 experimental groups. Control group (n=8): These animals are treated with the equivalent volume of PBS as used for the administration of Con A and D-GalN/LPS. Control hesperetin group (n=8): The mice are treated with hesperetin 400 mg/kg p.o. in 0.5% sodium carboxymethylcellulose (CMC-Na) solution for 10 days. Con A group (n=15): The animals are treated with the same volume of CMC-Na as used for administration of hesperetin for 10 days and are challenged with Con A (i.v.15 mg/kg). Con A + hesperetin groups: The animals receive various doses of hesperetin (100, 200, 400 mg/kg) p.o. for 10 days before Con A injection (each group n=15). D-GalN/LPS group (n=15): The animals are given CMC-Na for 10 days and injected i.p. with D-GalN (700 mg/kg)/LPS (5 μg/kg). D-GalN/LPS + hesperetin groups: Three doses of hesperetin (100, 200, 400 mg/kg) are given to mice once daily for 10 days. D-GalN (700 mg/kg)/LPS (5 μg/kg) are injected i.p. (each group n=15).
|
| References |
[1]. Arya A, et al. Bioflavonoid hesperetin overcome bicalutamide induced toxicity by co-delivery in novel SNEDDS formulations: Optimization, in vivo evaluation and uptake mechanism. Mater Sci Eng C Mater Biol Appl. 2017 Feb 1;71:954-964. [2]. Liu D, et al. Inhibitory Effect of Hesperetin and Naringenin on Human UDP-Glucuronosyltransferase Enzymes: Implications for Herb-Drug Interactions. Biol Pharm Bull. 2016;39(12):2052-2059. [3]. Oo A, et al. In silico study on anti-Chikungunya virus activity of hesperetin. PeerJ. 2016 Oct 26;4:e2602. eCollection 2016. [4]. Shagirtha K, et al. Neuroprotective efficacy of hesperetin against cadmium induced oxidative stress in the brain of rats. Toxicol Ind Health. 2016 Nov 1. pii: 0748233716665301. [5]. Bai X, et al. The protective effect of the natural compound hesperetin against fulminant hepatitis in vivo and in vitro. Br J Pharmacol. 2017 Jan;174(1):41-56.
|

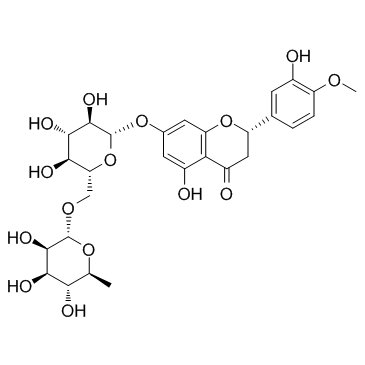 CAS#:520-26-3
CAS#:520-26-3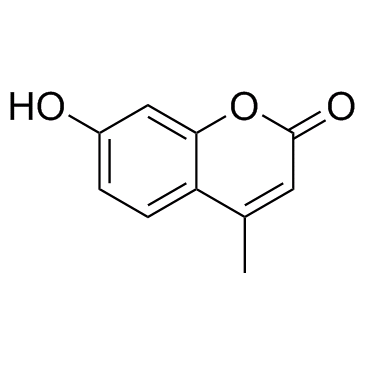 CAS#:90-33-5
CAS#:90-33-5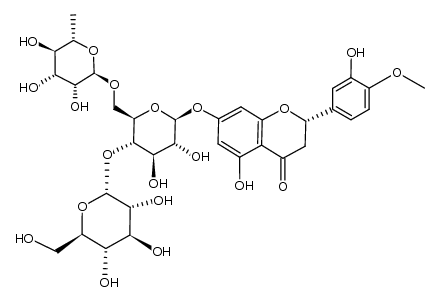 CAS#:161713-86-6
CAS#:161713-86-6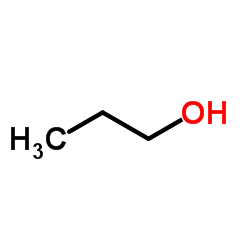 CAS#:71-23-8
CAS#:71-23-8 CAS#:64-17-5
CAS#:64-17-5 CAS#:71-41-0
CAS#:71-41-0 CAS#:67-63-0
CAS#:67-63-0 CAS#:100-51-6
CAS#:100-51-6 CAS#:35400-60-3
CAS#:35400-60-3 CAS#:603-61-2
CAS#:603-61-2 CAS#:520-34-3
CAS#:520-34-3 CAS#:17060-20-7
CAS#:17060-20-7 CAS#:70411-27-7
CAS#:70411-27-7![potassium 3-[5-hydroxy-2-(3-hydroxy-4-methoxy-phenyl)-4-oxo-chromen-7- yl]oxypropane-1-sulfonate structure](https://image.chemsrc.com/caspic/255/70412-86-1.png) CAS#:70412-86-1
CAS#:70412-86-1
