medroxyprogesterone
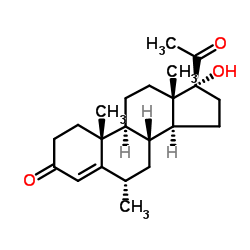
medroxyprogesterone structure
|
Common Name | medroxyprogesterone | ||
|---|---|---|---|---|
| CAS Number | 520-85-4 | Molecular Weight | 344.488 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 488.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C22H32O3 | Melting Point | 220-223.5ºC | |
| MSDS | Chinese USA | Flash Point | 263.0±25.2 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of medroxyprogesteroneMedroxyprogesterone is a progestin, a synthetic variant of the human hormone progesterone and a potent progesterone receptor agonist.Target: Progesterone ReceptorMedroxyprogesterone (MP), is a steroidal progestin drug which was never marketed for use in humans. An acylated derivative, medroxyprogesterone acetate (MPA), is clinically used as a pharmaceutical medicine. Compared to MPA, MP is over two orders of magnitude less potent as a progestogen. As such, MP itself is not used clinically, though it has seen limited use in veterinary medicine under the trade name Controlestril in France. In addition, it is an metabolite of MPA [1]. |
| Name | medroxyprogesterone |
|---|---|
| Synonym | More Synonyms |
| Description | Medroxyprogesterone is a progestin, a synthetic variant of the human hormone progesterone and a potent progesterone receptor agonist.Target: Progesterone ReceptorMedroxyprogesterone (MP), is a steroidal progestin drug which was never marketed for use in humans. An acylated derivative, medroxyprogesterone acetate (MPA), is clinically used as a pharmaceutical medicine. Compared to MPA, MP is over two orders of magnitude less potent as a progestogen. As such, MP itself is not used clinically, though it has seen limited use in veterinary medicine under the trade name Controlestril in France. In addition, it is an metabolite of MPA [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 488.0±45.0 °C at 760 mmHg |
| Melting Point | 220-223.5ºC |
| Molecular Formula | C22H32O3 |
| Molecular Weight | 344.488 |
| Flash Point | 263.0±25.2 °C |
| Exact Mass | 344.235138 |
| PSA | 54.37000 |
| LogP | 3.38 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.554 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | 48-63-68 |
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU5300000 |
| Precursor 6 | |
|---|---|
| DownStream 3 | |
|
Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts.
Springerplus 4 , 444, (2015) Clinical trials and epidemiological evidence have shown that combined estrogen/progestin hormone replacement therapy, but not estrogen therapy alone, increases breast cancer risk in post-menopausal wo... |
|
|
Development of a suspect and non-target screening approach to detect veterinary antibiotic residues in a complex biological matrix using liquid chromatography/high-resolution mass spectrometry.
Rapid Commun. Mass Spectrom. 29 , 2361-73, (2015) Swine manure can contain a wide range of veterinary antibiotics, which could enter the environment via manure spreading on agricultural fields. A suspect and non-target screening method was applied to... |
|
|
Anti-Human Immunodeficiency Virus Activity of Thiol-Ene Carbosilane Dendrimers and Their Potential Development as a Topical Microbicide.
J. Biomed. Nanotechnol. 11 , 1783-98, (2015) The concept of a "microbicide" was born out of the lack of a vaccine against HIV and the difficulty of women in ensuring the use of preventive prophylaxis by their partners, especially in developing c... |
| Medroxyprogesteron |
| Medroxyprogesteronum |
| Hydroxymethylprogesterone |
| MFCD00069474 |
| 17α-Hydroxy-6α-methylprogesterone |
| Medroxy Progesterone |
| 17-Hydroxy-6α-methylprogesterone |
| Medrossiprogesterone |
| 17alpha-Hydroxy-6alpha-methylprogesterone |
| (6a)-17-Hydroxy-6-methylpregn-4-ene-3,20-dione |
| 17-Hydroxy-6a-methylprogesterone |
| Medroxiprogesterone |
| 17a-Hydroxy-6a-methylprogesterone |
| 17-Hydroxy-6alpha-methylprogesterone |
| 6a-Methyl-17a-hydroxyprogesterone |
| 6α-Methyl-17α-hydroxyprogesterone |
| Farlutal |
| 17α-Hydroxy-6α-methylpregn-4-ene-3,20-dione |
| EINECS 208-298-6 |
| (6α)-17-Hydroxy-6-methylpregn-4-ene-3,20-dione |
| Pregn-4-ene-3,20-dione, 17-hydroxy-6α-methyl- |
| medroxyprogesterone |
| (6S,8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-6,10,13-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one |
| Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6α)- |
| Medroxiprogesteronum |
| Pregn-4-ene-3,20-dione, 17α-hydroxy-6α-methyl- |
| Medroxyprogesterone Base |
 CAS#:113665-92-2
CAS#:113665-92-2 CAS#:3386-01-4
CAS#:3386-01-4 CAS#:3386-00-3
CAS#:3386-00-3 CAS#:3496-78-4
CAS#:3496-78-4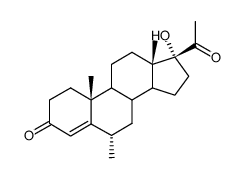 CAS#:80996-18-5
CAS#:80996-18-5 CAS#:1863-39-4
CAS#:1863-39-4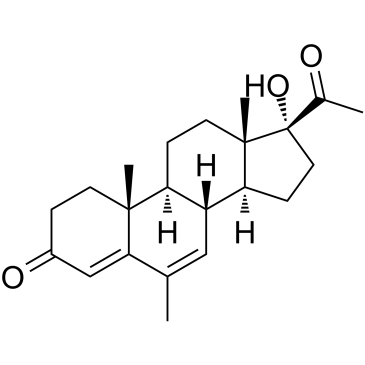 CAS#:3562-63-8
CAS#:3562-63-8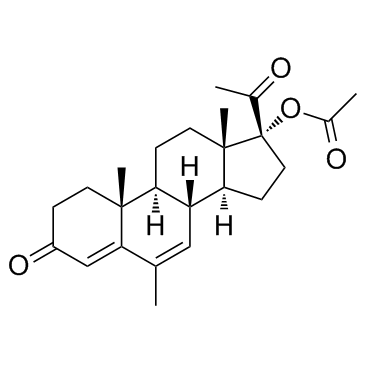 CAS#:595-33-5
CAS#:595-33-5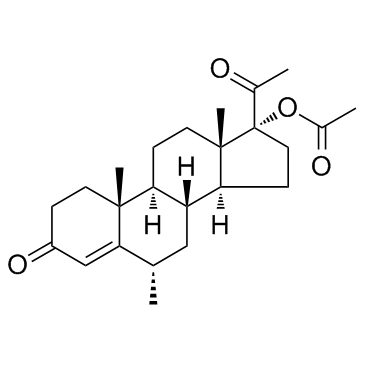 CAS#:71-58-9
CAS#:71-58-9