AFMK

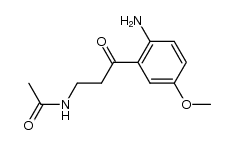
AFMK structure
|
Common Name | AFMK | ||
|---|---|---|---|---|
| CAS Number | 52450-38-1 | Molecular Weight | 264.277 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 589.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C13H16N2O4 | Melting Point | 138-140ºC | |
| MSDS | N/A | Flash Point | 310.3±30.1 °C | |
Use of AFMKAFMK, antioxidant metabolite of Melatonin, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. AFMK is a poorer scavenger. The pKa of AFMK at physiological pH is 8.7. Antioxidant capacity[1][2]. AFMK improves the anti-tumor effect of Gemcitabine in PANC-1 cells through the modulation of apoptotic pathway[3]. |
| Name | N-γ-Acetyl-N-2-formyl-5-methoxykynurenamine |
|---|---|
| Synonym | More Synonyms |
| Description | AFMK, antioxidant metabolite of Melatonin, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. AFMK is a poorer scavenger. The pKa of AFMK at physiological pH is 8.7. Antioxidant capacity[1][2]. AFMK improves the anti-tumor effect of Gemcitabine in PANC-1 cells through the modulation of apoptotic pathway[3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | AFMK is one of the metabolites of melatonin and can be formed by both enzymatic or pseudoenzymatic and nonenzymatic metabolic pathways[1]. AFMK pretreatment significantly inhibits DNA damage. AFMK shows a very high level of in vitro hydroxyl radical scavenging potential which was measured by an electron spin resonance (ESR) study. IC50 values resulting from ESR analysis was 338.08 nM. AFMK, a melatonin metabolite, is a sparingly investigated biogenic amine[2]. AFMK administered to PANC-1 in combination with Gemcitabine inhibits the production of HSP70 and cIAP-2 as compared to the results obtained with Gemcitabine alone[3]. Western Blot Analysis[3] Cell Line: Human pancreatic carcinoma cell line (PANC-1) Concentration: 0.001, 0.1, 10, 1000 nM Incubation Time: Result: Augmented the inhibitory effects on HSP70 expression from 0.47 (Gemcitabine alone) to 0.13 (10 nM AFMK), 0.08 (0.1 nM AFMK) and 0.01 (0.001 nM AFMK). |
| In Vivo | AFMK is a potent antioxidant in vivo. AFMK significantly reverses radiation-induced decline in the total antioxidant capacity of plasma in mice[2]. Animal Model: Male C57BL mice 8 wk of age[2] Dosage: 10 mg/kg body weight Administration: Intraperitoneal injection Result: Radiation-induced decline in the total antioxidant capacity of plasma was significantly reversed in AFMK pretreated mice. AFMK-pretreated irradiated groups showed a significantly lower value of comet tail length and % DNA in tail. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 589.4±50.0 °C at 760 mmHg |
| Melting Point | 138-140ºC |
| Molecular Formula | C13H16N2O4 |
| Molecular Weight | 264.277 |
| Flash Point | 310.3±30.1 °C |
| Exact Mass | 264.110992 |
| PSA | 84.50000 |
| LogP | 0.82 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.560 |
| InChIKey | JYWNYMJKURVPFH-UHFFFAOYSA-N |
| SMILES | COc1ccc(NC=O)c(C(=O)CCNC(C)=O)c1 |
| Storage condition | -20°C |
|
~63% 
AFMK CAS#:52450-38-1 |
| Literature: Amiet, Gary; Huegel, Helmut M.; Nurlawis, Faizul Synlett, 2002 , # 3 p. 495 - 497 |
|
~% 
AFMK CAS#:52450-38-1 |
| Literature: Harthe, Catherine; Claudy, Daniele; Dechaud, Henri; Vivien-Roels, Berthe; Pevet, Paul; Claustrat, Bruno Life Sciences, 2003 , vol. 73, # 12 p. 1587 - 1597 |
|
~% 
AFMK CAS#:52450-38-1 |
| Literature: Amiet, Gary; Huegel, Helmut M.; Nurlawis, Faizul Synlett, 2002 , # 3 p. 495 - 497 |
|
~% 
AFMK CAS#:52450-38-1 |
| Literature: Amiet, Gary; Huegel, Helmut M.; Nurlawis, Faizul Synlett, 2002 , # 3 p. 495 - 497 |
|
~% 
AFMK CAS#:52450-38-1 |
| Literature: Collin, Fabrice; Bonnefont-Rousselot, Dominique; Yous, Said; Marchetti, Catherine; Jore, Daniel; Gardes-Albert, Monique Journal of Mass Spectrometry, 2009 , vol. 44, # 3 p. 318 - 329 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| N-[3-(2-Formamido-5-methoxyphenyl)-3-oxopropyl]acetamide |
| Acetamide, N-[3-[2-(formylamino)-5-methoxyphenyl]-3-oxopropyl]- |