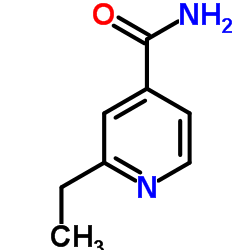
Ethionamide

Ethionamide structure
|
Common Name | Ethionamide | ||
|---|---|---|---|---|
| CAS Number | 536-33-4 | Molecular Weight | 166.24300 | |
| Density | 1.17 g/cm3 | Boiling Point | 167 °C / 1mmHg | |
| Molecular Formula | C8H10N2S | Melting Point | 164 °C | |
| MSDS | USA | Flash Point | 133.2ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of EthionamideEthionamide(2-ethylthioisonicotinamide) is an antibiotic used in the treatment of tuberculosis.Target: AntibacterialEthionamide is a second-line antitubercular agent that inhibits mycolic acid synthesis. It also may be used for treatment of leprosy. Ethionamide is a prodrug. It is activated by the enzyme EthA, a mono-oxygenase in Mycobacterium tuberculosis, and binds NAD+ to form an adduct which inhibits InhA in the same way as isoniazid. Expression of the ethA gene is controlled by EthR, a transcriptional repressor. It is understood that improving ethA expression will increase the efficacy of ethionamide and so EthR inhibitors are of great interest to co-drug developers. The action may be through disruption of mycolic acid [1, 2]. |
| Name | ethionamide |
|---|---|
| Synonym | More Synonyms |
| Description | Ethionamide(2-ethylthioisonicotinamide) is an antibiotic used in the treatment of tuberculosis.Target: AntibacterialEthionamide is a second-line antitubercular agent that inhibits mycolic acid synthesis. It also may be used for treatment of leprosy. Ethionamide is a prodrug. It is activated by the enzyme EthA, a mono-oxygenase in Mycobacterium tuberculosis, and binds NAD+ to form an adduct which inhibits InhA in the same way as isoniazid. Expression of the ethA gene is controlled by EthR, a transcriptional repressor. It is understood that improving ethA expression will increase the efficacy of ethionamide and so EthR inhibitors are of great interest to co-drug developers. The action may be through disruption of mycolic acid [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.17 g/cm3 |
|---|---|
| Boiling Point | 167 °C / 1mmHg |
| Melting Point | 164 °C |
| Molecular Formula | C8H10N2S |
| Molecular Weight | 166.24300 |
| Flash Point | 133.2ºC |
| Exact Mass | 166.05600 |
| PSA | 71.00000 |
| LogP | 1.97850 |
| Index of Refraction | 1.599 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H361 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R63 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NS0350000 |
| HS Code | 2933990090 |
|
~80% 
Ethionamide CAS#:536-33-4 |
| Literature: Bergman, Jan; Pettersson, Birgitta; Hasimbegovic, Vedran; Svensson, Per H. Journal of Organic Chemistry, 2011 , vol. 76, # 6 p. 1546 - 1553 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Bulletin de la Societe Chimique de France, , p. 687,691 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Bulletin de la Societe Chimique de France, , p. 687,691 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Bulletin de la Societe Chimique de France, , p. 687,691 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Zhurnal Obshchei Khimii, , vol. 29, p. 915,918; engl. Ausg. S. 898 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Zhurnal Obshchei Khimii, , vol. 29, p. 915,918; engl. Ausg. S. 898 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Zhurnal Obshchei Khimii, , vol. 29, p. 915,918; engl. Ausg. S. 898 |
|
~% 
Ethionamide CAS#:536-33-4 |
| Literature: Zhurnal Obshchei Khimii, , vol. 29, p. 915,918; engl. Ausg. S. 898 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The efflux pump inhibitor timcodar improves the potency of antimycobacterial agents.
Antimicrob. Agents Chemother. 59(3) , 1534-41, (2015) Previous studies indicated that inhibition of efflux pumps augments tuberculosis therapy. In this study, we used timcodar (formerly VX-853) to determine if this efflux pump inhibitor could increase th... |
|
|
Simple and accurate quantitative analysis of 20 anti-tuberculosis drugs in human plasma using liquid chromatography-electrospray ionization-tandem mass spectrometry.
J. Pharm. Biomed. Anal. 102 , 9-16, (2014) A simple and accurate liquid chromatography (LC)-tandem mass spectrometry (MS/MS) method for the quantitation of 20 anti-tuberculosis (anti-TB) drugs in human plasma, was developed as a tool for thera... |
|
|
Comparative study of the effects of antituberculosis drugs and antiretroviral drugs on cytochrome P450 3A4 and P-glycoprotein.
Antimicrob. Agents Chemother. 58(6) , 3168-76, (2014) Predicting drug-drug interactions (DDIs) related to cytochrome P450 (CYP), such as CYP3A4 and one of the major drug transporters, P-glycoprotein (P-gp), is crucial in the development of future chemoth... |
| 2-ethyl-4-thiopyridylamide |
| 2-ethyl-4-thiocarbamoylpyridine |
| EINECS 208-628-9 |
| Ethionamide |
| 2-Ethyl-4-pyridinecarbothioamide |
| Etionamid |
| 2-Ethyl-4-pyridinecarbothioamide,Ethionamide |
| Etioniamid |
| Trecator |
| Ethyonomide |
| Thioamide |
| Ethioniamide |
| Ethinamide |
| Ethylisothiamide |
| 2-Ethylpyridine-4-carbothioamide |
| 2-ethyl-4-thiocarbamoyl-4-pyridine |
| MFCD00057361 |
| Trecator-SC |
| 2-Ethylthioisonicotinamide |