(+)-Cloprostenol
Modify Date: 2024-01-02 20:01:03
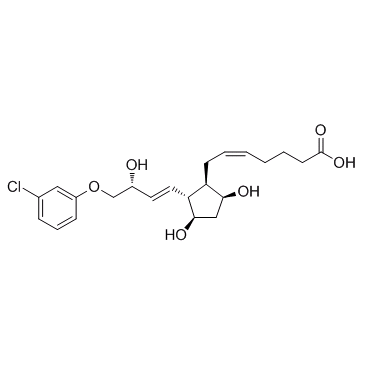
(+)-Cloprostenol structure
|
Common Name | (+)-Cloprostenol | ||
|---|---|---|---|---|
| CAS Number | 54276-21-0 | Molecular Weight | 424.915 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 628.0±55.0 °C at 760 mmHg | |
| Molecular Formula | C22H29ClO6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 333.6±31.5 °C | |
Use of (+)-Cloprostenol(+)-Cloprostenol is a prostaglandin F2α (PGF2α) analogue, and shows selective agonistic activity at the prostaglandin receptor. |
| Name | (+)-Cloprostenol |
|---|---|
| Synonym | More Synonyms |
| Description | (+)-Cloprostenol is a prostaglandin F2α (PGF2α) analogue, and shows selective agonistic activity at the prostaglandin receptor. |
|---|---|
| Related Catalog | |
| Target |
PGF2α |
| In Vitro | D-Cloprostenol and PGF2 alpha are equipotent, about 150 times more potent than dl-cloprostenol (P < 0.05) and approximately 280 times more potent than PGE1 in inhibiting [3H]PGF2 alpha binding to corpus luteum cell membranes. However, d-cloprostenol and PGF2 alpha are about 10 times more potent than dl-cloprostenol and approximately 95 times more potent than PGE1 in myometrial cell membranes[2]. |
| In Vivo | D-cloprostenol (15 g per head) is the lowest dose that consistently achieves abortion; D-cloprostenol causes mild adverse effects including salivation, defecation and hyperventilation in bitches weighing less than 10 kg. Intra-vesicle administration of a single low dose of d-cloprostenol is a safe and successful technique to induce abortion in the bitch[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 628.0±55.0 °C at 760 mmHg |
| Molecular Formula | C22H29ClO6 |
| Molecular Weight | 424.915 |
| Flash Point | 333.6±31.5 °C |
| Exact Mass | 424.165253 |
| PSA | 110.05000 |
| LogP | 2.31 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.623 |
| 5-Heptenoic acid, 7-((1R,2R,3R,5S)-2-((1E,3R)-4-(3-chlorophenoxy)-3-hydroxy-1-butenyl)-3,5-dihydroxycyclopentyl)-, (5Z)-rel- |
| 5-Heptenoic acid, 7-[(1R,2R,3R,5S)-2-[(1E,3R)-4-(3-chlorophenoxy)-3-hydroxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl]-, (5Z)- |
| 5-Heptenoic acid, 7-((1R,2R,3R,5S)-2-((1E,3R)-4-(3-chlorophenoxy)-3-hydroxy-1-butenyl)-3,5-dihydroxycyclopentyl)-, monosodium salt, (5Z)-rel- |
| (±)-Sodium (Z)-7-((1R*,2R*,3R*,5S*)-2-((E)-(3R*)-4-(m-Chlorophenoxy)-3-hydroxy-1-butenyl)-3,5-dihydroxycyclopentyl)-5-heptenoate |
| Cloprostenol |
| Oestrophan |
| Sodium (5Z)-7-{(1R,2R,3R,5S)-2-[(1E,3R)-4-(3-chlorophenoxy)-3-hydroxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl}-5-heptenoate |
| (Z)-7-[(1S,2S,3S,5R)-2-[(E,3S)-4-(3-chloranylphenoxy)-3-oxidanyl-but-1-enyl]-3,5-bis(oxidanyl)cyclopentyl]hept-5-enoic acid |
| Dalmazin [veterinary] (TN) |
| Racemic cloprostenol |
| Estrophan |
| (Z)-7-(2-((E)-4-(3-chlorophenoxy)-3-hydroxybut-1-enyl)-3,5-dihydroxycyclopentyl)hept-5-enoic acid |
| (5Z)-rel-7-((1R,2R,3R,5S)-2-((1E,3R)-4-(3-Chlorophenoxy)-3-hydroxy-1-butenyl)-3,5-dihydroxycyclopentyl)-5-heptenoic Acid Monosodium Salt |
| (5Z)-7-{(1R,2R,3R,5S)-2-[(1E,3R)-4-(3-Chlorophenoxy)-3-hydroxybut-1-en-1-yl]-3,5-dihydroxycyclopentyl}hept-5-enoic Acid |
| Sodium (5Z)-7-{(1R,2R,3R,5S)-2-[(1E,3R)-4-(3-chlorophenoxy)-3-hydroxybut-1-en-1-yl]-3,5-dihydroxycyclopentyl}hept-5-enoate |
| Cloprostenol Sodium |
| 5-Heptenoic acid, 7-[(1R,2R,3R,5S)-2-[(1E,3R)-4-(3-chlorophenoxy)-3-hydroxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl]-, sodium salt, (5Z)- (1:1) |
| (5Z)-7-{(1R,2R,3R,5S)-2-[(1E,3R)-4-(3-Chlorophenoxy)-3-hydroxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl}-5-heptenoic acid |
| Estrofan |
| UNII:4208238832 |
| Cloprostenol (INN) |
| UNII:886SAV9675 |
| (Z)-7-[(1S,2S,3S,5R)-2-[(E,3S)-4-(3-chlorophenoxy)-3-hydroxybut-1-enyl]-3,5-dihydroxycyclopentyl]-5-heptenoic acid |
 CAS#:32233-40-2
CAS#:32233-40-2 CAS#:62961-72-2
CAS#:62961-72-2![4-[4-(3-chlorophenoxy)-3-oxobut-1-enyl]-5-hydroxy-3,3a,4,5,6,6a-hexahydrocyclopenta[b]furan-2-one Structure](https://www.chemsrc.com/caspic/284/53872-62-1.png) CAS#:53872-62-1
CAS#:53872-62-1 CAS#:40665-92-7
CAS#:40665-92-7 CAS#:41723-91-5
CAS#:41723-91-5