Epirubicin
Modify Date: 2025-08-21 13:33:26
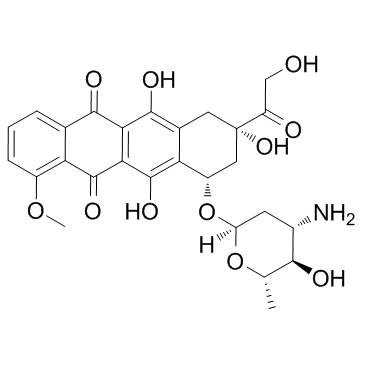
Epirubicin structure
|
Common Name | Epirubicin | ||
|---|---|---|---|---|
| CAS Number | 56420-45-2 | Molecular Weight | 543.519 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 810.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C27H29NO11 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 443.8±34.3 °C | |
Use of EpirubicinEpirubicin is a semisynthetic L-arabino derivative of doxorubicin, and an antineoplastic agent by inhibiting Topoisomerase. |
| Name | 4'-epidoxorubicin |
|---|---|
| Synonym | More Synonyms |
| Description | Epirubicin is a semisynthetic L-arabino derivative of doxorubicin, and an antineoplastic agent by inhibiting Topoisomerase. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase |
| In Vitro | Epirubicin, like doxorubicin, exerts its antitumor effects by complex with DNA, resulting in damage to DNA and interference with the synthesis of DNA, RNA, and proteins. Epirubicin may also affect the integrity and activity of cellular membranes. Maximal cell kill caused by Epirubicin occurs during the S phase of the cell cycle. With higher concentrations effects are also seen in early G2 as well as G1 and M phases[1]. Epirubicin display antineoplastic activity against most cancer cells. Epirubicin is cytotoxic to Hepatoma G2 cells with IC50 of 1.6 μg/mL at 24 hr. 1.6 μg/mL Epirubicin induces apoptosis of Hep G2 cells, and higher activity of catalase by 50%, Se-dependent glutathione peroxidase by 110%, Cu, Zn-superoxide dismutase by 172% and Mn-superoxide dismutase by 135%. Epirbicin increases the cellular expression of NADPH-CYP 450 reductase, and reduces GST-π expression[2]. |
| In Vivo | Epirubicin are clinically active against a broad range of tumor types, including breast cancer, malignant lymphomas, soft tissue sarcomas, lung cancer, pleural mesothelioma, gastrointestinal cancer, head and neck cancer, ovarian cancer, prostatic carcinoma, transitional bladder carcinoma and so on[3]. Epirubicin at a dose of 3.5 mg/kg suppresses tumor mass of human breast tumor xenograft R-27 by 74.4 %[4]. |
| Cell Assay | Hep G2 cells (500 cells/well, monolayer) are plated in a 96-well plate. The next day the cells are treated with Epirubicin in the medium. At the end of the incubation periods, 15% volume of MTT dye solution is added. After 1 hr of incubation at 37°C, an equal volume of solubilization/stop solution (dimethylsul-foxide) is added to each well for an additional 1 hr incubation. The absorbance of the reaction solution at 570 nm is recorded. |
| References |
[3]. Bonadonna, G., et al. Drugs ten years later: epirubicin. Ann Oncol, 1993. 4(5): p. 359-69. |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 810.3±65.0 °C at 760 mmHg |
| Molecular Formula | C27H29NO11 |
| Molecular Weight | 543.519 |
| Flash Point | 443.8±34.3 °C |
| Exact Mass | 543.174072 |
| PSA | 206.07000 |
| LogP | 2.82 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.710 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside |
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-a-L-arabino-hexopyranoside |
| (8S-cis)-10-[(3-Amino-2,3,6-trideoxy-a-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione |
| farmorubicin |
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside |
| 4'-EPIDOXORUBICIN |
| 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)- |
| 3-Glycoloyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-tridioxy-a-L-arabinohexopyranoside |
| epi-dx |
| Epirubicin |
| 4'-epi-DX |
| 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S)- |
| WP 697 |
| 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)- |
| Pidorubicin |
| Epirubicin Ebewe |
| Epiadriamycin |
| (1S,3S)-3-Glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside |
| 4'-Epidoxorubiein |
| 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)- |
| Pharmorubicin |
| imi28 |
| Adriblastin |
| 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-a-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)- |
| (3S)-3-Glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside |
| (1S,3S)-3-Glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl 3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranoside |
| Farmarubicin |
| 4'-epiadriamycin |