Cefmetazole Sodium
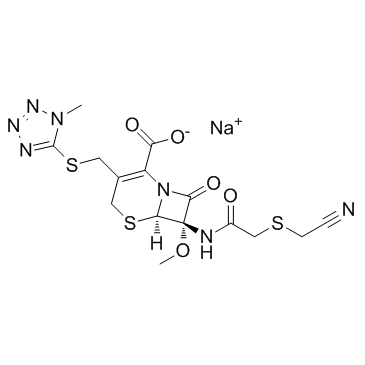
Cefmetazole Sodium structure
|
Common Name | Cefmetazole Sodium | ||
|---|---|---|---|---|
| CAS Number | 56796-39-5 | Molecular Weight | 493.516 | |
| Density | 1.75 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H16N7NaO5S3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Cefmetazole SodiumCefmetazole sodium is a semisynthetic cephamycin antibiotic. Target: AntibacterialCefmetazole sodium has a broad spectrum of activity comparable to that of the second-generation cephalosporins, covering gram-positive, gram-negative, and anaerobic bacteria. Unlike the second-generation cephalosporins, cephamycins such as cefmetazole are usually active against Bacteroides fragilis. Cefmetazole is also active against beta-lactamase-producing organisms that are resistant to first-generation cephalosporins or penicillins. The pharmacokinetics of cefmetazole allow parenteral administration (intravenous or intramuscular) 2-3 times daily for treatment of infection. The drug has been studied in gynecologic, intraabdominal, urinary tract, respiratory tract, and skin and soft tissue infections. Administered preoperatively, it may reduce the frequency of infection in certain clean-contaminated or potentially contaminated procedures, including cesarean section, abdominal or vaginal hysterectomy, cholecystectomy (high-risk patients), and colorectal surgery. |
| Name | cefmetazole sodium |
|---|---|
| Synonym | More Synonyms |
| Description | Cefmetazole sodium is a semisynthetic cephamycin antibiotic. Target: AntibacterialCefmetazole sodium has a broad spectrum of activity comparable to that of the second-generation cephalosporins, covering gram-positive, gram-negative, and anaerobic bacteria. Unlike the second-generation cephalosporins, cephamycins such as cefmetazole are usually active against Bacteroides fragilis. Cefmetazole is also active against beta-lactamase-producing organisms that are resistant to first-generation cephalosporins or penicillins. The pharmacokinetics of cefmetazole allow parenteral administration (intravenous or intramuscular) 2-3 times daily for treatment of infection. The drug has been studied in gynecologic, intraabdominal, urinary tract, respiratory tract, and skin and soft tissue infections. Administered preoperatively, it may reduce the frequency of infection in certain clean-contaminated or potentially contaminated procedures, including cesarean section, abdominal or vaginal hysterectomy, cholecystectomy (high-risk patients), and colorectal surgery. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.75 g/cm3 |
|---|---|
| Molecular Formula | C15H16N7NaO5S3 |
| Molecular Weight | 493.516 |
| Exact Mass | 493.027283 |
| PSA | 242.06000 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H317-H319-H334-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338-P342 + P311 |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38;R42/43 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | XI0372200 |
|
Chimeric mice with humanized liver.
Toxicology 246(1) , 9-17, (2008) Recently, chimeric mice with humanized liver were established by transplanting human hepatocytes into an urokinase-type plasminogen activator(+/+)/severe combined immunodeficient transgenic mouse line... |
|
|
Usefulness of sennoside as an agent for mechanical bowel preparation prior to elective colon cancer surgery
Asian J. Surg. 35(2) , 81-7, (2012) Objective We retrospectively evaluated the usefulness of sennoside as an agent for mechanical bowel preparation prior to elective colon cancer surgery. |
|
|
Involvement of multidrug resistance-associated protein 4 in efflux transport of prostaglandin E(2) across mouse blood-brain barrier and its inhibition by intravenous administration of cephalosporins.
J. Pharmacol. Exp. Ther. 333 , 912-919, (2010) Prostaglandin E(2) (PGE(2)) acts as a modulator of synaptic signaling and excitability in the brain. Because PGE(2) is barely inactivated enzymatically in adult brain, its brain level is considered to... |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[2-[(cyanomethyl)thio]acetyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, sodium salt, (6R,7S)- (1:1) |
| sodium,(6R,7S)-7-[[2-(cyanomethylsulfanyl)acetyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Sodium (6R,7S)-7-(2-((Cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| MFCD00865068 |
| Cefmetazole Sodium Salt |
| Sodium (6R,7S)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Cefmetazole (sodium) |

