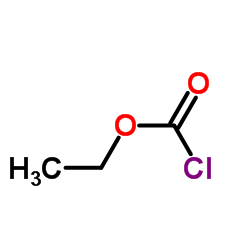
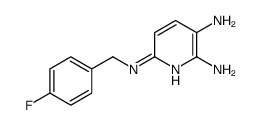
Flupirtine
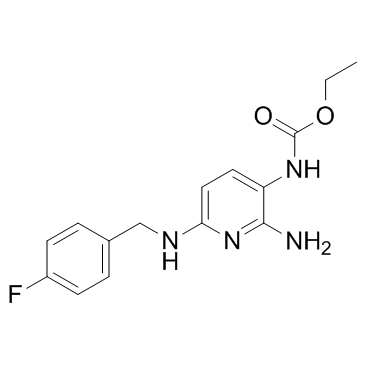
Flupirtine structure
|
Common Name | Flupirtine | ||
|---|---|---|---|---|
| CAS Number | 56995-20-1 | Molecular Weight | 304.31900 | |
| Density | 1.35g/cm3 | Boiling Point | 434.9ºC at 760mmHg | |
| Molecular Formula | C15H17FN4O2 | Melting Point | 115 - 116ºC | |
| MSDS | USA | Flash Point | 216.8ºC | |
Use of FlupirtineFlupirtine(D 9998) is a selective neuronal potassium channel opener that also has NMDA receptor antagonist properties.IC50 Value: Target: Potassium channel; NMDA receptorin vitro: High concentrations of flupirtine antagonized inward currents to NMDA(200 microM) at -70 mV with an lC50 against steady-state responses of 182.1+/-12.1 microM. The effects of flupirtine were voltage-independent and not associated with receptor desensitization making actions within the NMDA receptor channel or at the glycine modulatory site unlikely. NMDA receptor antagonism probably has little relevance for the clinical efficacy of flupirtine as the concentrations needed were far higher than those achieved in clinical practice. However, the activation of a G-protein-regulated inwardly rectifying K+ channel was identified as an interesting molecular target site of flupirtine. In the next stage, the central nervous spectrum of action of experimental K+ channel openers (PCO) was considered. As far as they have been studied, experimental K+ channel openers display a spectrum of action comparable to that of flupirtine [1]. Therapeutic flupirtine concentrations (≤10 ?M) did not affect voltage-gated Na(+) or Ca(2+) channels, inward rectifier K(+) channels, nicotinic acetylcholine receptors, glycine or ionotropic glutamate receptors. Flupirtine shifted the gating of K(V)7 K(+) channels to more negative potentials and the gating of GABA(A) receptors to lower GABA concentrations [2]. Cell exposure to flupirtine decreased the amplitude of delayed rectifier K(+) current (I(K(DR))) with a concomitant raise in current inactivation in NSC-34 neuronal cells [4].in vivo: Rats were trained to discriminate the novel analgesic flupirtine (10.0 mg/kg i.p., 10 min) from no drug under a two-choice fixed-ratio 5 shock-termination schedule. Flupirtine yielded a dose-response curve with an ED50 of 3.87 mg/kg. The opioid analgesics pentazocine, codeine and tramadol failed to produce flupirtine appropriate responding. The opioid antagonist naltrexone did not antagonize the discriminative effects of flupirtine [3]. Both morphine (ED?? =?0.74?mg/kg) and flupirtine (ED???=?3.32?mg/kg) caused dose-related anti-hyperalgesia at doses that did not cause sedation [5]. Toxicity: Based on study-end data, hepatotoxicity was detected in 31% of patients receiving flupirtine for ≥ 6 weeks [6]. |
| Name | ethyl N-[2-amino-6-[(4-fluorophenyl)methylamino]pyridin-3-yl]carbamate |
|---|---|
| Synonym | More Synonyms |
| Description | Flupirtine(D 9998) is a selective neuronal potassium channel opener that also has NMDA receptor antagonist properties.IC50 Value: Target: Potassium channel; NMDA receptorin vitro: High concentrations of flupirtine antagonized inward currents to NMDA(200 microM) at -70 mV with an lC50 against steady-state responses of 182.1+/-12.1 microM. The effects of flupirtine were voltage-independent and not associated with receptor desensitization making actions within the NMDA receptor channel or at the glycine modulatory site unlikely. NMDA receptor antagonism probably has little relevance for the clinical efficacy of flupirtine as the concentrations needed were far higher than those achieved in clinical practice. However, the activation of a G-protein-regulated inwardly rectifying K+ channel was identified as an interesting molecular target site of flupirtine. In the next stage, the central nervous spectrum of action of experimental K+ channel openers (PCO) was considered. As far as they have been studied, experimental K+ channel openers display a spectrum of action comparable to that of flupirtine [1]. Therapeutic flupirtine concentrations (≤10 ?M) did not affect voltage-gated Na(+) or Ca(2+) channels, inward rectifier K(+) channels, nicotinic acetylcholine receptors, glycine or ionotropic glutamate receptors. Flupirtine shifted the gating of K(V)7 K(+) channels to more negative potentials and the gating of GABA(A) receptors to lower GABA concentrations [2]. Cell exposure to flupirtine decreased the amplitude of delayed rectifier K(+) current (I(K(DR))) with a concomitant raise in current inactivation in NSC-34 neuronal cells [4].in vivo: Rats were trained to discriminate the novel analgesic flupirtine (10.0 mg/kg i.p., 10 min) from no drug under a two-choice fixed-ratio 5 shock-termination schedule. Flupirtine yielded a dose-response curve with an ED50 of 3.87 mg/kg. The opioid analgesics pentazocine, codeine and tramadol failed to produce flupirtine appropriate responding. The opioid antagonist naltrexone did not antagonize the discriminative effects of flupirtine [3]. Both morphine (ED?? =?0.74?mg/kg) and flupirtine (ED???=?3.32?mg/kg) caused dose-related anti-hyperalgesia at doses that did not cause sedation [5]. Toxicity: Based on study-end data, hepatotoxicity was detected in 31% of patients receiving flupirtine for ≥ 6 weeks [6]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.35g/cm3 |
|---|---|
| Boiling Point | 434.9ºC at 760mmHg |
| Melting Point | 115 - 116ºC |
| Molecular Formula | C15H17FN4O2 |
| Molecular Weight | 304.31900 |
| Flash Point | 216.8ºC |
| Exact Mass | 304.13400 |
| PSA | 89.27000 |
| LogP | 3.71060 |
| Index of Refraction | 1.661 |
| InChIKey | JUUFBMODXQKSTD-UHFFFAOYSA-N |
| SMILES | CCOC(=O)Nc1ccc(NCc2ccc(F)cc2)nc1N |
| Storage condition | 2-8℃ |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
Flupirtine CAS#:56995-20-1 |
| Literature: Helvetica Chimica Acta, , vol. 77, # 8 p. 2175 - 2190 |
|
~% 
Flupirtine CAS#:56995-20-1 |
| Literature: Helvetica Chimica Acta, , vol. 77, # 8 p. 2175 - 2190 |
|
~% 
Flupirtine CAS#:56995-20-1 |
| Literature: Helvetica Chimica Acta, , vol. 77, # 8 p. 2175 - 2190 |
|
~% 
Flupirtine CAS#:56995-20-1 |
| Literature: Helvetica Chimica Acta, , vol. 77, # 8 p. 2175 - 2190 |
| EINECS 260-503-8 |
| Flupirtine |
| Effirma |

![spiro[1,3-dihydroimidazo[4,5-b]pyridine-2,1'-cyclohexane] structure](https://image.chemsrc.com/caspic/102/76902-24-4.png)