benzocatechol
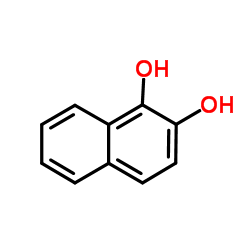
benzocatechol structure
|
Common Name | benzocatechol | ||
|---|---|---|---|---|
| CAS Number | 574-00-5 | Molecular Weight | 160.169 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 353.9±15.0 °C at 760 mmHg | |
| Molecular Formula | C10H8O2 | Melting Point | 101-103 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 181.0±15.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | naphthalene-1,2-diol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 353.9±15.0 °C at 760 mmHg |
| Melting Point | 101-103 °C(lit.) |
| Molecular Formula | C10H8O2 |
| Molecular Weight | 160.169 |
| Flash Point | 181.0±15.0 °C |
| Exact Mass | 160.052429 |
| PSA | 40.46000 |
| LogP | 2.11 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.726 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2907299090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2907299090 |
|---|---|
| Summary | 2907299090 polyphenols; phenol-alcohols。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:5.5%。general tariff:30.0% |
|
Identifying chelators for metalloprotein inhibitors using a fragment-based approach.
J. Med. Chem. 54 , 591-602, (2011) Fragment-based lead design (FBLD) has been used to identify new metal-binding groups for metalloenzyme inhibitors. When screened at 1 mM, a chelator fragment library (CFL-1.1) of 96 compounds produced... |
|
|
Selective antiproliferative activity of hydroxynaphthyl-beta-D-xylosides.
J. Med. Chem. 49 , 1932-8, (2006) The antiproliferative activity of the 14 isomeric monoxylosylated dihydroxynaphthalenes has been tested in vitro toward normal HFL-1 and 3T3 A31 cells as well as transformed T24 and 3T3 SV40 cells. Th... |
|
|
Metabolism of acenaphthylene via 1,2-dihydroxynaphthalene and catechol by Stenotrophomonas sp. RMSK.
Biodegradation 20(6) , 837-43, (2009) Stenotrophomonas sp. RMSK capable of degrading acenaphthylene as a sole source of carbon and energy was isolated from coal sample. Metabolites produced were analyzed and characterized by TLC, HPLC and... |
| 1,2-Naphthalenediol |
| benzocatechol |
| Naphthalene-1,2-diol |
| MFCD00003959 |
| EINECS 209-365-2 |
| 1,2-Dihydroxynaphthalene |
| dihydroxynaphthalene |
| naphthalenediol |
 CAS#:708-06-5
CAS#:708-06-5 CAS#:135-19-3
CAS#:135-19-3 CAS#:524-42-5
CAS#:524-42-5 CAS#:90-15-3
CAS#:90-15-3 CAS#:771-16-4
CAS#:771-16-4 CAS#:6336-79-4
CAS#:6336-79-4 CAS#:1344113-50-3
CAS#:1344113-50-3 CAS#:30432-52-1
CAS#:30432-52-1 CAS#:91-20-3
CAS#:91-20-3 CAS#:1663-45-2
CAS#:1663-45-2 CAS#:83-72-7
CAS#:83-72-7![(Z)-3,3'-dihydroxy-4H,4'H-[1,1'-binaphthalenylidene]-4,4'-dione structure](https://image.chemsrc.com/caspic/362/76364-88-0.png) CAS#:76364-88-0
CAS#:76364-88-0 CAS#:123-31-9
CAS#:123-31-9![3',4'-dihydroxy-[1,1']binaphthyl-3,4-dione structure](https://image.chemsrc.com/caspic/147/60599-29-3.png) CAS#:60599-29-3
CAS#:60599-29-3 CAS#:2834-92-6
CAS#:2834-92-6 CAS#:16223-99-7
CAS#:16223-99-7![3,3-dimethyl-1,2-dihydrobenzo[f]chromene structure](https://image.chemsrc.com/caspic/439/14472-44-7.png) CAS#:14472-44-7
CAS#:14472-44-7![3H-Naphtho[2,1-b]pyran, 3,3-dimethyl- structure](https://image.chemsrc.com/caspic/094/19836-62-5.png) CAS#:19836-62-5
CAS#:19836-62-5 CAS#:18515-10-1
CAS#:18515-10-1