UNII:4FM1N296CM
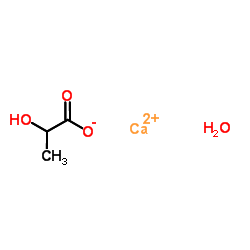
UNII:4FM1N296CM structure
|
Common Name | UNII:4FM1N296CM | ||
|---|---|---|---|---|
| CAS Number | 5743-47-5 | Molecular Weight | 308.294 | |
| Density | N/A | Boiling Point | 227.6ºC at 760 mmHg | |
| Molecular Formula | C6H20CaO11 | Melting Point | 240 °C | |
| MSDS | USA | Flash Point | 109.9ºC | |
| Name | L-Calcium lactate |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 227.6ºC at 760 mmHg |
|---|---|
| Melting Point | 240 °C |
| Molecular Formula | C6H20CaO11 |
| Molecular Weight | 308.294 |
| Flash Point | 109.9ºC |
| Exact Mass | 308.063141 |
| PSA | 166.87000 |
| Storage condition | 2-8°C |
| Water Solubility | soluble |
Synonym:(S)-2-Hydroxypropanioc acid calcium salt pentahydrate; Lactic acid calcium salt pentahydrate Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Not available. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. Low hazard for usual industrial handling. Ingestion: Ingestion of large amounts may cause gastrointestinal irritation. Inhalation: May cause respiratory tract irritation. Low hazard for usual industrial handling. Chronic: No information found. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid. Inhalation: Remove from exposure and move to fresh air immediately. Get medical aid if cough or other symptoms appear. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. This material in sufficient quantity and reduced particle size is capable of creating a dust explosion. Runoff from fire control or dilution water may cause pollution. Extinguishing Media: For small fires, use water spray, dry chemical, carbon dioxide or chemical foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with skin and eyes. Avoid ingestion and inhalation. Storage: Store in a cool, dry place. Keep from contact with oxidizing materials. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 5743-47-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: white Odor: No odor. pH: Not available. Vapor Pressure: Negligible. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 240 deg C Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: 9 g/100ml in water (25 c) Specific Gravity/Density: Molecular Formula: C6H10O6Ca.5H2O Molecular Weight: 308.29 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable at room temperature in closed containers under normal storage and handling conditions. Conditions to Avoid: Dust generation, excess heat. Incompatibilities with Other Materials: Strong oxidizers. Hazardous Decomposition Products: Carbon monoxide, carbon dioxide, calcium oxide. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 5743-47-5 unlisted. LD50/LC50: Not available. Carcinogenicity: Calcium lactate, pentahydrate - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 5743-47-5: 0 Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 5743-47-5 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 5743-47-5 is not on the TSCA Inventory because it is a hydrate. It is considered to be listed if the CAS number for the anhydrous form is on the inventory (40CFR720.3(u)(2)). SECTION 16 - ADDITIONAL INFORMATION N/A |
|
Synthesis of 45S5 Bioglass® via a straightforward organic, nitrate-free sol-gel process.
Mater. Sci. Eng. C. Mater. Biol. Appl. 40 , 248-52, (2014) More than four decades after the discovery of 45S5 Bioglass® as the first bioactive material, this composition is still one of the most promising materials in the tissue engineering field. Sol-gel-der... |
|
|
Spontaneous supersaturation of calcium D-gluconate during isothermal dissolution of calcium L-lactate in aqueous sodium D-gluconate.
Food Funct. 5(1) , 85-91, (2014) Continuing dissolution of solid calcium L-lactate pentahydrate in saturated aqueous solutions following addition of solid sodium D-gluconate corresponding to a gluconate/lactate ratio around three was... |
|
|
In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model.
PLoS ONE 6(11) , e27452, (2011) Phthalates are ubiquitous environmental contaminants because of their use in plastics and other common consumer products. Di-(2-ethylhexyl) phthalate (DEHP) is the most abundant phthalate and it impai... |
| calcium,2-hydroxypropanoate,pentahydrate |
| Propanoic acid, 2-hydroxy-, calcium salt, hydrate (2:1:5) |
| MFCD00287281 |
| UNII:4FM1N296CM |
| EINECS 248-953-3 |
| Calcium 2-hydroxypropanoate hydrate (1:2:5) |
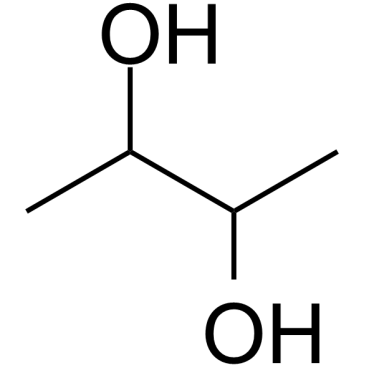 CAS#:513-85-9
CAS#:513-85-9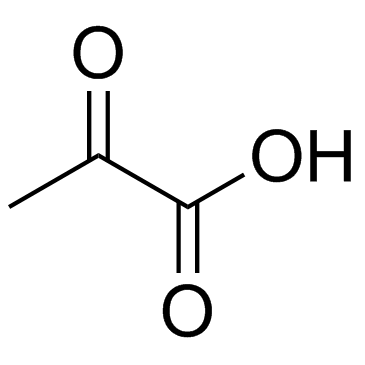 CAS#:127-17-3
CAS#:127-17-3 CAS#:64-17-5
CAS#:64-17-5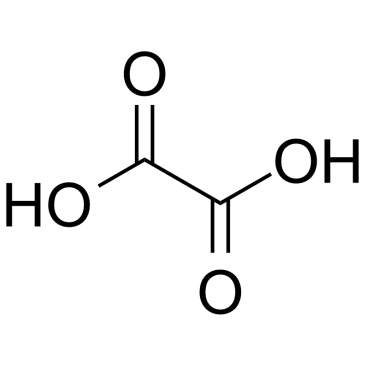 CAS#:144-62-7
CAS#:144-62-7 CAS#:64-19-7
CAS#:64-19-7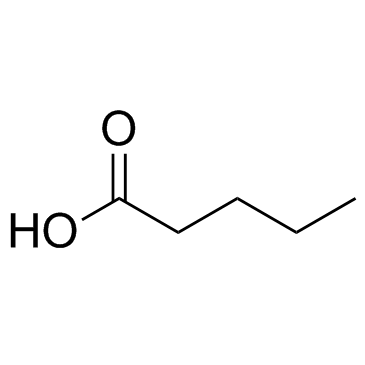 CAS#:109-52-4
CAS#:109-52-4 CAS#:64-18-6
CAS#:64-18-6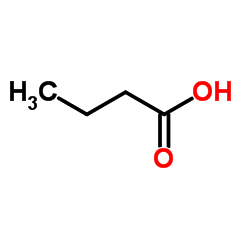 CAS#:107-92-6
CAS#:107-92-6 CAS#:300-85-6
CAS#:300-85-6
