CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
GU5290000
-
CHEMICAL NAME :
-
2,5-Cyclohexadiene-1,4-dione, 5,6-dimethoxy-2-(10-hydroxydecyl)-3-methyl-
-
CAS REGISTRY NUMBER :
-
58186-27-9
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C19-H30-O5
-
MOLECULAR WEIGHT :
-
338.49
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
10 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Lungs, Thorax, or Respiration - other changes Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3931,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
830 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3931,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3931,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3931,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
757 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3931,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3931,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CPBTAL Chemical and Pharmaceutical Bulletin. (Japan Pub. Trading Co., USA, 1255 Howard St., San Francisco, CA 94103) V.6- 1958- Volume(issue)/page/year: 39,367,1991 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
17500 mg/kg/5W-C
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,3955,1985
|
 CAS#:58186-28-0
CAS#:58186-28-0 CAS#:1251831-79-4
CAS#:1251831-79-4 CAS#:77712-29-9
CAS#:77712-29-9 CAS#:55362-80-6
CAS#:55362-80-6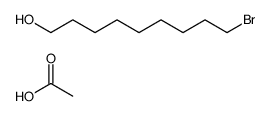 CAS#:53596-82-0
CAS#:53596-82-0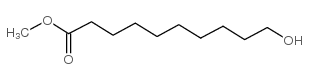 CAS#:2640-94-0
CAS#:2640-94-0 CAS#:92038-37-4
CAS#:92038-37-4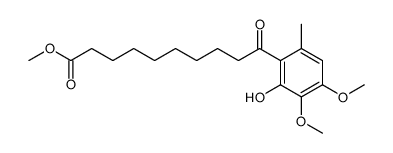 CAS#:58185-88-9
CAS#:58185-88-9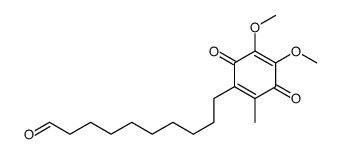 CAS#:89048-27-1
CAS#:89048-27-1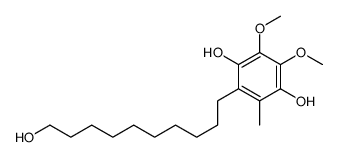 CAS#:58186-26-8
CAS#:58186-26-8