Toyocamycin
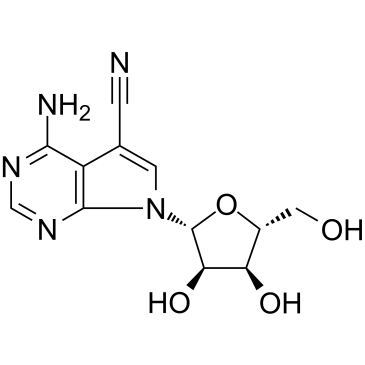
Toyocamycin structure
|
Common Name | Toyocamycin | ||
|---|---|---|---|---|
| CAS Number | 606-58-6 | Molecular Weight | 291.26300 | |
| Density | 1.91g/cm3 | Boiling Point | 721.1ºC at 760 mmHg | |
| Molecular Formula | C12H13N5O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 389.9ºC | |
Use of ToyocamycinToyocamycin (Vengicide) is an adenosine analog produced by Actinomycete, acts as an XBP1 inhibitor, inhibits IRE1α-induced ATP-dependent XBP1 mRNA cleavage, with an IC50 of 80 nM. Toyocamycin (Vengicide) induces apoptosis. Toyocamycin (Vengicide) shows no effect on IRE1α auto-phosphorylation[1]. |
| Name | 4-AMINO-5-CYANO-7-(β-D-RIBOFURANOSYL)PYRROLO[2,3-D]PYRIMIDINE |
|---|---|
| Synonym | More Synonyms |
| Description | Toyocamycin (Vengicide) is an adenosine analog produced by Actinomycete, acts as an XBP1 inhibitor, inhibits IRE1α-induced ATP-dependent XBP1 mRNA cleavage, with an IC50 of 80 nM. Toyocamycin (Vengicide) induces apoptosis. Toyocamycin (Vengicide) shows no effect on IRE1α auto-phosphorylation[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 80 nM (XBP1 activation)[1] |
| References |
| Density | 1.91g/cm3 |
|---|---|
| Boiling Point | 721.1ºC at 760 mmHg |
| Molecular Formula | C12H13N5O4 |
| Molecular Weight | 291.26300 |
| Flash Point | 389.9ºC |
| Exact Mass | 291.09700 |
| PSA | 150.44000 |
| Index of Refraction | 1.849 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Synthesis of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides. Isosteres of sangivamycin, tubercidin, and toyocamycin.
Carbohydr. Res. 331(1) , 77-82, (2001) Syntheses of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides are reported. Treatment of pyranulose glycoside with aminoguanidine in acetic acid gave the corresponding semicarbazone in 96% yield. The ring ... |
|
|
Synthesis of 2'-beta-C-methyl toyocamycin and sangivamycin analogues as potential HCV inhibitors.
Bioorg. Med. Chem. Lett. 15(3) , 725-7, (2005) Coupling reaction of 2-beta-C-methyl-1,2,3,4-tetra-O-benzoyl-d-ribofuranose with 4-amino-6-bromo-5-cyanopyrrolo[2,3-d]pyrimidine, followed by debromination and debenzoylation, gave the 2'-beta-C-methy... |
|
|
Cell cycle arrest and cytochrome c-mediated apoptotic induction in human lung cancer A549 cells by MCS-C2, an analogue of sangivamycin.
J. Microbiol. Biotechnol. 20(2) , 433-7, (2010) In the course of our screening for novel modulators on cell cycle progression and apoptosis as anticancer drug candidates, we generated an analogue of sangivamycin, MCS-C2, designated as 4-amino-6-bro... |
| TOYOCAMYCIN |
| vengicide |
| siromycin |
| a-399-y4 |
| unamycin-b |
| e-212 |
| uramycinb |
| 7-Cyano-7-deazaadenosine |
| e-212-1 |
![5-chloro-9-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2,4,9-triazabicyclo[4.3.0]nona-2,4,7,10-tetraene-7-carbonitrile structure](https://image.chemsrc.com/caspic/495/52443-16-0.png) CAS#:52443-16-0
CAS#:52443-16-0