Oxatomide
Oxatomide structure
|
Common Name | Oxatomide | ||
|---|---|---|---|---|
| CAS Number | 60607-34-3 | Molecular Weight | 426.55300 | |
| Density | 1.175 g/cm3 | Boiling Point | 621.1ºC at 760 mmHg | |
| Molecular Formula | C27H30N4O | Melting Point | 153.60C | |
| MSDS | Chinese USA | Flash Point | 329.4ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of OxatomideOxatomide is a potent and orally active dual H1-histamine receptor and P2X7 receptor antagonist with antihistamine and anti-allergic activity. Oxatomide almost completely blocks the ATP-induced current in human P2X7 receptors (IC50 of 0.95 μM). Oxatomide inhibits ATP-induced Ca2+ influx with an IC50 value of 0.43 μM and also inhibits serotonin[1][2]. |
| Name | 3-[3-(4-benzhydrylpiperazin-1-yl)propyl]-1H-benzimidazol-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | Oxatomide is a potent and orally active dual H1-histamine receptor and P2X7 receptor antagonist with antihistamine and anti-allergic activity. Oxatomide almost completely blocks the ATP-induced current in human P2X7 receptors (IC50 of 0.95 μM). Oxatomide inhibits ATP-induced Ca2+ influx with an IC50 value of 0.43 μM and also inhibits serotonin[1][2]. |
|---|---|
| Related Catalog | |
| Target |
H1 Receptor P2X7 serotonin |
| References |
| Density | 1.175 g/cm3 |
|---|---|
| Boiling Point | 621.1ºC at 760 mmHg |
| Melting Point | 153.60C |
| Molecular Formula | C27H30N4O |
| Molecular Weight | 426.55300 |
| Flash Point | 329.4ºC |
| Exact Mass | 426.24200 |
| PSA | 44.27000 |
| LogP | 4.00270 |
| Index of Refraction | 1.619 |
| InChIKey | BAINIUMDFURPJM-UHFFFAOYSA-N |
| SMILES | O=c1[nH]c2ccccc2n1CCCN1CCN(C(c2ccccc2)c2ccccc2)CC1 |
| Storage condition | Refrigerator |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DE2276000 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Treatment of allergic rhinitis in infants and children: efficacy and safety of second-generation antihistamines and the leukotriene receptor antagonist montelukast.
Drugs 69(18) , 2541-76, (2009) Allergic rhinitis (AR) affects a large percentage of paediatric patients. With the wide array of available agents, it has become a challenge to choose the most appropriate treatment for patients. Seco... |
|
|
Differential thermodynamic driving force of first- and second-generation antihistamines to determine their binding affinity for human H1 receptors.
Biochem. Pharmacol. 91(2) , 231-41, (2014) Differential binding sites for first- and second-generation antihistamines were indicated on the basis of the crystal structure of human histamine H1 receptors. In this study, we evaluated differences... |
|
|
Molecular complexes of ketaconazole and oxatomide with p-chloranil: spectroscopic and spectrofluorimetric studies.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 79(5) , 1137-44, (2011) The molecular complexes of the donors ketaconazole (KTZ) and oxatomide (OXA) drugs with 2,3,5,6-tetrachloro-1,4-benzoquinone (p-chloranil, p-CHL) have been investigated spectroscopically (UV-vis, FT-I... |
| Oxatomidum |
| MFCD00211147 |
| KW-4354 |
| EINECS 262-320-9 |
| Oxatomida |
| Oxatomidum [INN-Latin] |
| oxatomide |
| 1-[3-[4-(Diphenylmethyl)-1-piperazinyl]propyl]-1,3-dihydro-2H-benzimidazol-2-one |
| Oxetal |
| Tinset |
| Oxatimide |
| Celtect |
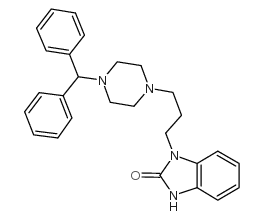
 CAS#:841-77-0
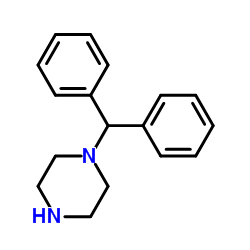
CAS#:841-77-0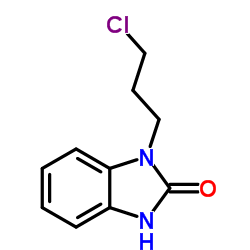 CAS#:62780-89-6
CAS#:62780-89-6 CAS#:62781-00-4
CAS#:62781-00-4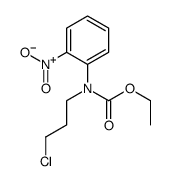 CAS#:100460-82-0
CAS#:100460-82-0![N-ethoxycarbonyl-N-[3-(4-diphenylmethylpiperazin-1-yl)prop-1-yl]-o-nitroaniline Structure](https://image.chemsrc.com/caspic/043/100460-83-1.png) CAS#:100460-83-1
CAS#:100460-83-1![N-ethoxycarbonyl-N-[3-(4-diphenylmethylpiperazin-1-yl)prop-1-yl]-o-phenylenediamine Structure](https://image.chemsrc.com/caspic/429/100460-86-4.png) CAS#:100460-86-4
CAS#:100460-86-4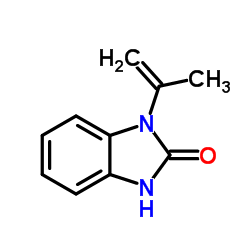 CAS#:52099-72-6
CAS#:52099-72-6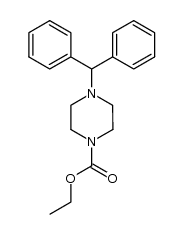 CAS#:102552-76-1
CAS#:102552-76-1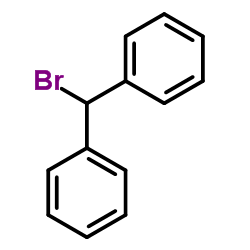 CAS#:776-74-9
CAS#:776-74-9 CAS#:100460-85-3
CAS#:100460-85-3