Acteoside
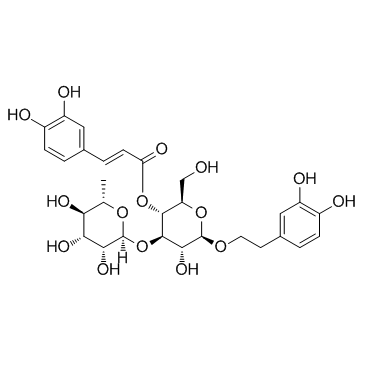
Acteoside structure
|
Common Name | Acteoside | ||
|---|---|---|---|---|
| CAS Number | 61276-17-3 | Molecular Weight | 624.587 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 908.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C29H36O15 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 294.7±27.8 °C | |
Use of ActeosideVerbascoside is isolated from Lantana camara, acts as an ATP-competitive inhibitor of PKC, with an IC50 of 25 µM, and has antitumor, anti-inflammatory and antineuropathic pain activity. |
| Name | Verbascoside |
|---|---|
| Synonym | More Synonyms |
| Description | Verbascoside is isolated from Lantana camara, acts as an ATP-competitive inhibitor of PKC, with an IC50 of 25 µM, and has antitumor, anti-inflammatory and antineuropathic pain activity. |
|---|---|
| Related Catalog | |
| Target |
PKC:25 μM (IC50) |
| In Vitro | Verbascoside acts as an ATP-competitive inhibitor of PKC, with an IC50 of 25 µM. Verbascoside shows Kis of 22 and 28 µM with respect to ATP and histone, respectively. Verbascoside has potent antitumor activity against L-1210 cells, with an IC50 of 13 µM[1]. Verbascoside (5, 10 µM) suppresses 2,4-dinitrochlorobenzene (DNCB)-induced T cell costimulatory factors CD86 and CD54, proinflammatory cytokines, and NFκB pathway activation in THP-1 cells[2]. |
| In Vivo | Verbascoside (1%) reduces the overall scratching behavior incidence as well as the severity of the skin lesions in 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis (AD) mice model. Verbascoside also blocks DNCB-induced expression of proinflammatory cytokine TNF-α, IL-6, and IL-4 mRNA in skin lesions[2]. Verbascoside (50, 100 mg/kg, i.p.) does not modify chronic constriction injury (CCI)-induced cold allodynia. Verbascoside (200 mg/kg, i.p.) decreases hyper-sensitivity to cold stimulus, acetone, on day 3 in rats. Verbascoside also significantly reduces behavioral changes associated with neuropathy. Moreover, Verbascoside decreases Bax and increases Bcl-2 on day 3[3]. |
| Cell Assay | The lymphocytic mouse leukemia L1210 cells (ATCC, CCL 219) are plated sparsely at 104 cells per well in 24-well cluster plates in Dulbecco’s modified Eagle medium containing 10% fetal calf serum, 4 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin sulfate, and Verbascoside (solubilized in DMSO). After a 2-day incubation period at 37°C in a humidified atmosphere (5% CO2 in air), growth is monitored by counting cell numbers in a Coulter-counter. IC50 values are calculated on the basis of the linear regression lines established for each compound tested[1]. |
| Animal Admin | Rats[2] To induce atopic dermatitis (AD)-like symptoms, 2,4-dinitrochlorobenzene (DNCB) is used. Briefly, the dorsal hair of the mice is removed using an electronic clipper 2 days before DNCB treatment. An application of 200 µL of 1% DNCB (in acetone:olive oil = 4:1) is made to the shaved dorsal skin for sensitization. The repeated challenge is performed on the same site with 0.2% DNCB once every 3 days for about 2 weeks. The mice are divided into 4 groups (n = 6 per group): (1) vehicle-treated controls, (2) DNCB-treated only, (3) 1% Verbascoside (in acetone:olive oil 4:1)-treated only, and (4) DNCB + 1% Verbascoside-treated group[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 908.8±65.0 °C at 760 mmHg |
| Molecular Formula | C29H36O15 |
| Molecular Weight | 624.587 |
| Flash Point | 294.7±27.8 °C |
| Exact Mass | 624.205444 |
| PSA | 245.29000 |
| LogP | 2.44 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.689 |
| InChIKey | FBSKJMQYURKNSU-FSIAKBNXSA-N |
| SMILES | CC1OC(OC2C(O)C(OCCc3ccc(O)c(O)c3)OC(CO)C2OC(=O)C=Cc2ccc(O)c(O)c2)C(O)C(O)C1O |
| Storage condition | room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | LZ5786000 |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
|
Modulation of human keratinocyte responses to solar UV by plant polyphenols as a basis for chemoprevention of non-melanoma skin cancers.
Curr. Med. Chem. 20(7) , 869-79, (2013) Excessive exposure to solar UVA and UVB radiation is widely considered to cause skin cancers such as squamous cell carcinoma and basalioma. Direct UVB damage to skin cell DNA as well as UV-induced chr... |
|
|
A metabolite-profiling approach to assess the uptake and metabolism of phenolic compounds from olive leaves in SKBR3 cells by HPLC-ESI-QTOF-MS.
J. Pharm. Biomed. Anal. 72 , 121-6, (2013) Olive leaves, an easily available natural low-cost material, constitute a source of extracts with significant antitumor activity that inhibits cell proliferation in several breast-cancer-cell models. ... |
|
|
Acylated phenylethanoid glycosides, echinacoside and acteoside from Cistanche tubulosa, improve glucose tolerance in mice.
J. Nat. Med. 68(3) , 561-6, (2014) Acylated phenylethanoid glycosides, echinacoside (1) and acteoside (2), principal constituents in stems of Cistanche tubulosa (Orobanchaceae), inhibited the increase in postprandial blood glucose leve... |
| Acteoside |
| β-D-Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl 3-O-(6-deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]- |
| (2R,3R,4R,5R,6R)-6-[2-(3,4-Dihydroxyphenyl)ethoxy]-5-hydroxy-2-(hydroxymethyl)-4-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-3-yl-(2E)-3-(3,4-dihydroxyphenyl)acrylat |
| 2-(3,4-Dihydroxyphenyl)ethyl 3-O-(6-deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-β-D-glucopyranoside |
| β-D-Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl 3-O-(6-deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]- |
| T6OTJ B1 CQ DQ EQ FO- DT6OTJ BO2R CQ DQ& CQ EOV1U1R CQ DQ& F1Q &&Stereoisomer |
| verbascoside |
| 2-(3,4-Dihydroxyphenyl)ethyl-3-O-(6-deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-β-D-glucopyranoside |
| (2E)-3-(3,4-Dihydroxyphényl)acrylate de (2R,3R,4R,5R,6R)-6-[2-(3,4-dihydroxyphényl)éthoxy]-5-hydroxy-2-(hydroxyméthyl)-4-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}tétrahydro-2H-pyran-3-yle |
| (2R,3R,4R,5R,6R)-6-[2-(3,4-Dihydroxyphenyl)ethoxy]-5-hydroxy-2-(hydroxymethyl)-4-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-3-yl (2E)-3-(3,4-dihydroxyphenyl)acrylate |
| MFCD06798947 |
| 3-O-(6-Désoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphényl)-2-propènan-1-oyl]-β-D-glucopyranoside de 2-(3,4-dihydroxyphényl)éthyle |
| 2-(3,4-Dihydroxyphenyl)ethyl 3-O-(6-deoxy-α-L-mannopyranosyl)-4-O-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]-β-D-glucopyranoside |
 CAS#:253790-76-0
CAS#:253790-76-0 CAS#:96826-11-8
CAS#:96826-11-8![3-[3,4-bis(phenylmethoxy)phenyl]prop-2-enoic acid Structure](https://image.chemsrc.com/caspic/028/54429-62-8.png) CAS#:54429-62-8
CAS#:54429-62-8 CAS#:39698-00-5
CAS#:39698-00-5 CAS#:253790-67-9
CAS#:253790-67-9 CAS#:253790-69-1
CAS#:253790-69-1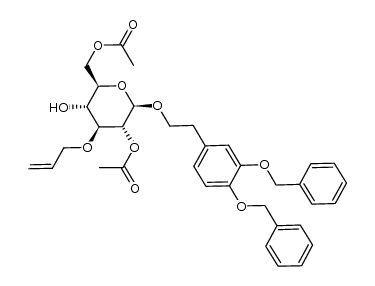 CAS#:253790-72-6
CAS#:253790-72-6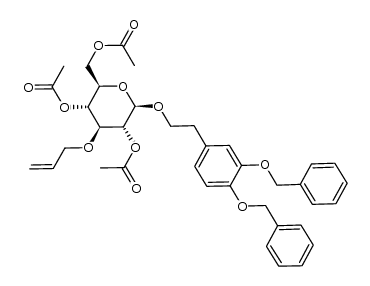 CAS#:253790-68-0
CAS#:253790-68-0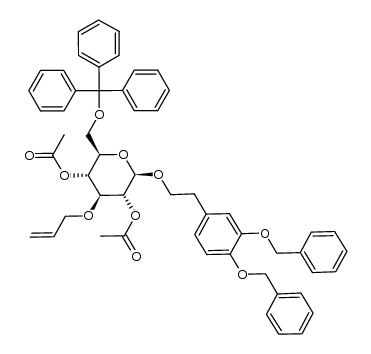 CAS#:253790-71-5
CAS#:253790-71-5 CAS#:10597-60-1
CAS#:10597-60-1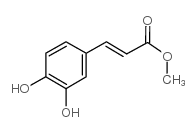 CAS#:3843-74-1
CAS#:3843-74-1 CAS#:61303-13-7
CAS#:61303-13-7
