6 β hydroxy testosterone
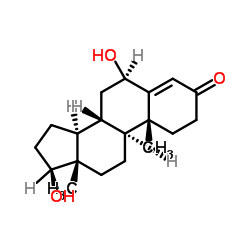
6 β hydroxy testosterone structure
|
Common Name | 6 β hydroxy testosterone | ||
|---|---|---|---|---|
| CAS Number | 62-99-7 | Molecular Weight | 304.424 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 479.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H28O3 | Melting Point | ≥205ºC | |
| MSDS | Chinese USA | Flash Point | 258.1±25.2 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
| Name | 6beta-hydroxytestosterone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 479.8±45.0 °C at 760 mmHg |
| Melting Point | ≥205ºC |
| Molecular Formula | C19H28O3 |
| Molecular Weight | 304.424 |
| Flash Point | 258.1±25.2 °C |
| Exact Mass | 304.203857 |
| PSA | 57.53000 |
| LogP | 1.86 |
| Appearance of Characters | white to off-white |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.578 |
| InChIKey | XSEGWEUVSZRCBC-ZVBLRVHNSA-N |
| SMILES | CC12CCC(=O)C=C1C(O)CC1C2CCC2(C)C(O)CCC12 |
| Storage condition | 2-8°C |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | 61-40 |
| Safety Phrases | 53-22-36/37/39 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
|
Testosterone-metabolizing capacity and characteristics of adrenal microsomes in human fetus in vitro.
J. Pediatr. Endocrinol. Metab. 23(1-2) , 143-52, (2010) We describe the metabolite spectrum of testosterone (T) in human fetal adrenal in vitro, and verify possible roles of CYP3A and 2C isoforms of human fetal adrenal in T metabolism which respond to T me... |
|
|
The hepatic and intestinal metabolic activities of P450 in rats with surgery- and drug-induced renal dysfunction.
Pharm. Res. 20(10) , 1591-4, (2003) The hepatic and intestinal metabolic activities of P450 were evaluated in rats with surgery- and drug-induced renal dysfunction.Renal failure was induced by five-sixths nephrectomy (NR), bilateral ure... |
|
|
The use of heat treatment to eliminate drug interactions due to grapefruit juice.
Biol. Pharm. Bull. 29(11) , 2274-8, (2006) Grapefruit juice (GJ) contains components that may increase the bioavailability of drugs; however, approaches to the removal of these components have been little investigated. It is known that furanoc... |
| 6,17-Dihydroxy-6b,17b-androst-4-en-3-one |
| 4-ANDROSTENE-6BETA,17BETA-DIOL-3-ONE |
| 6β,17β-Dihydroxyandrost-4-en-3-one |
| 6β-HYDROXYTESTOSTERONE |
| 6,17-dihydroxy-(6b,17b)-Androst-4-en-3-one |
| 6β- Hydroxytestosterone |
| 6-hydroxytestosterone |
| 6BETA-HYDROXYTESTOSERONE |
| 6,7-Dihydroxy-6b,17b-androst-4-en-one |
| (6β,17β)-6,17-Dihydroxyandrost-4-en-3-one |
| 4-Androsten-6b,17b-diol-3-one |
| 6,7-Dihydroxy-6,17-androst-4-en-one |
| Androst-4-en-3-one, 6,17-dihydroxy-, (6β,17β)- |
| 6,17-dihydroxy-(6β,17β)-Androst-4-en-3-one |
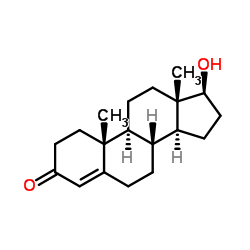 CAS#:58-22-0
CAS#:58-22-0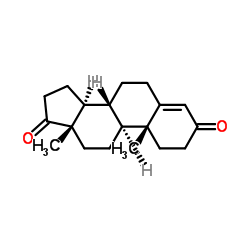 CAS#:63-05-8
CAS#:63-05-8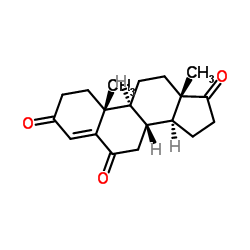 CAS#:2243-06-3
CAS#:2243-06-3