4-Aminobenzyl alcohol
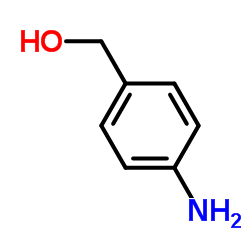
4-Aminobenzyl alcohol structure
|
Common Name | 4-Aminobenzyl alcohol | ||
|---|---|---|---|---|
| CAS Number | 623-04-1 | Molecular Weight | 123.15 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 284.8±15.0 °C at 760 mmHg | |
| Molecular Formula | C7H9NO | Melting Point | 60-65 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 126.0±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-Aminobenzyl alcohol4-Aminobenzyl alcohol is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Aminobenzyl alcohol |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Aminobenzyl alcohol is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 284.8±15.0 °C at 760 mmHg |
| Melting Point | 60-65 °C(lit.) |
| Molecular Formula | C7H9NO |
| Molecular Weight | 123.15 |
| Flash Point | 126.0±20.4 °C |
| Exact Mass | 123.068413 |
| PSA | 46.25000 |
| LogP | -0.25 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.617 |
| Storage condition | 2-8°C |
| Stability | Stable, but air and light sensitive. Incompatible with strong oxidizing agents, strong acids. Combustible. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922199090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
DNA-templated release of functional molecules with an azide-reduction-triggered immolative linker.
Chem. Commun. (Camb.) 47(15) , 4364-66, (2011) Nucleic acid templated reactions have attracted significant attention for nucleic acid sensing. Herein we report a general design which extends the potential of nucleic acid templated reactions to unl... |
|
|
Design, synthesis, and biological evaluation of a dual tumor-specific motive containing integrin-targeted plasmin-cleavable doxorubicin prodrug.
Mol. Cancer Ther. 1(11) , 901-11, (2002) The design, synthesis, and initial biological evaluation of a doxorubicin prodrug that contains a dual tumor specific moiety, which allows enhanced tumor recognition potential, is reported. Both a tum... |
|
|
Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems.
Biomaterials 23(9) , 1955-66, (2002) The design and preparation of novel biodegradable hydrogels developed by the free radical polymerization of acrylamide and acrylic acid, and some formulations with bis-acrylamide, in the presence of a... |
| 4-Aminobenzyl alcohol |
| para-aminobenzylalcohol |
| (4-Aminophenyl)methanol |
| Benzenemethanol, 4-amino- |
| Benzyl alcohol, p-amino- |
| EINECS 210-767-5 |
| MFCD00014782 |
| 4-aminobenzylalcohol |
 CAS#:555-16-8
CAS#:555-16-8 CAS#:556-18-3
CAS#:556-18-3 CAS#:619-73-8
CAS#:619-73-8![[4-(Hydroxymethyl)phenyl]boronic acid Structure](https://image.chemsrc.com/caspic/251/59016-93-2.png) CAS#:59016-93-2
CAS#:59016-93-2 CAS#:18282-51-4
CAS#:18282-51-4 CAS#:150-13-0
CAS#:150-13-0 CAS#:18484-05-4
CAS#:18484-05-4 CAS#:179806-93-0
CAS#:179806-93-0 CAS#:145153-06-6
CAS#:145153-06-6 CAS#:181819-75-0
CAS#:181819-75-0 CAS#:1703-46-4
CAS#:1703-46-4 CAS#:131230-76-7
CAS#:131230-76-7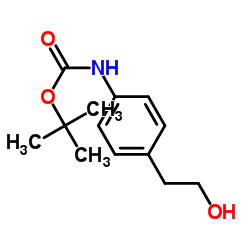 CAS#:104060-23-3
CAS#:104060-23-3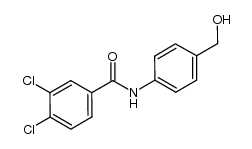 CAS#:1146717-36-3
CAS#:1146717-36-3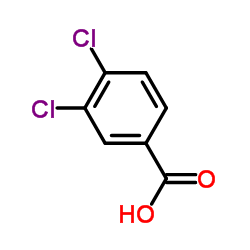 CAS#:51-44-5
CAS#:51-44-5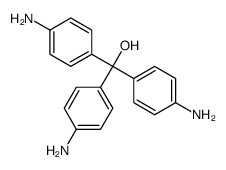 CAS#:467-62-9
CAS#:467-62-9 CAS#:13738-70-0
CAS#:13738-70-0
