Furan-2-ylmethyl acetate
Modify Date: 2025-08-23 14:46:51
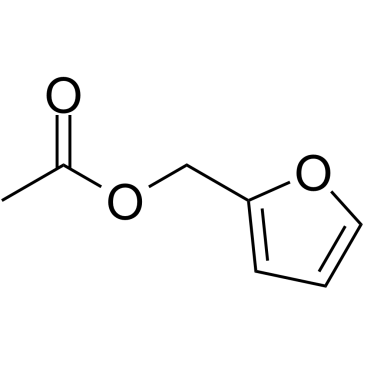
Furan-2-ylmethyl acetate structure
|
Common Name | Furan-2-ylmethyl acetate | ||
|---|---|---|---|---|
| CAS Number | 623-17-6 | Molecular Weight | 140.137 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 177.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C7H8O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 65.6±0.0 °C | |
Use of Furan-2-ylmethyl acetateFurfuryl acetate can be used in the synthesis of 5-acetoxymethyl-2-vinylfuran and 5-hydroxymethyl-2-vinylfuran[1]. |
| Name | Furfuryl acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Furfuryl acetate can be used in the synthesis of 5-acetoxymethyl-2-vinylfuran and 5-hydroxymethyl-2-vinylfuran[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 177.0±0.0 °C at 760 mmHg |
| Molecular Formula | C7H8O3 |
| Molecular Weight | 140.137 |
| Flash Point | 65.6±0.0 °C |
| Exact Mass | 140.047348 |
| PSA | 39.44000 |
| LogP | 1.09 |
| Vapour Pressure | 1.1±0.3 mmHg at 25°C |
| Index of Refraction | 1.464 |
| InChIKey | CKOYRRWBOKMNRG-UHFFFAOYSA-N |
| SMILES | CC(=O)OCc1ccco1 |
| Water Solubility | 0.5-1.0 g/100 mL at 23 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25-S37/39-S26 |
| WGK Germany | 3 |
| RTECS | LU9120000 |
| HS Code | 2932190090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932190090 |
|---|---|
| Summary | 2932190090 other compounds containing an unfused furan ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Synthesis of 5-acetoxymethyl- and 5-hydroxymethyl-2-vinyl-furan.
Molecules 12(3) , 634-40, (2007) 5-Acetoxymethyl- and 5-hydroxymethyl-2-vinylfuran were synthesized by two routes. The first route starts from 2-methylfuran and the second from furfuryl acetate. The latter route, involving successive... |
| LU9120000 [RTECS] |
| 2-(Acetoxymethyl)furan |
| Furan-2-ylmethyl acetate |
| Furfuryl acetate |
| MFCD00003251 |
| T5OJ B1OV1 |
| EINECS 210-775-9 |
| Furan-2-ylmethylacetat |
| 2-Furylmethyl acetate |
| 2-Furanmethanol, acetate |
| 2-Furylmethylacetat |
 CAS#:98-00-0
CAS#:98-00-0 CAS#:108-24-7
CAS#:108-24-7 CAS#:10226-94-5
CAS#:10226-94-5 CAS#:108-05-4
CAS#:108-05-4 CAS#:98-01-1
CAS#:98-01-1 CAS#:64-19-7
CAS#:64-19-7 CAS#:39110-68-4
CAS#:39110-68-4 CAS#:102-82-9
CAS#:102-82-9 CAS#:2846-62-0
CAS#:2846-62-0 CAS#:141-78-6
CAS#:141-78-6 CAS#:37444-46-5
CAS#:37444-46-5 CAS#:96-47-9
CAS#:96-47-9 CAS#:698-63-5
CAS#:698-63-5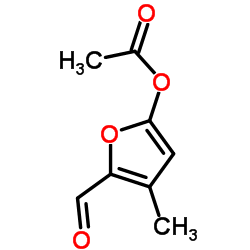 CAS#:10551-58-3
CAS#:10551-58-3 CAS#:16361-14-1
CAS#:16361-14-1 CAS#:2493-04-1
CAS#:2493-04-1 CAS#:13679-46-4
CAS#:13679-46-4![2-[(4-methoxyphenyl)methyl]furan structure](https://image.chemsrc.com/caspic/028/15047-76-4.png) CAS#:15047-76-4
CAS#:15047-76-4![(3-oxo-8-oxabicyclo[3.2.1]oct-6-en-5-yl)methyl acetate structure](https://image.chemsrc.com/caspic/359/69471-70-1.png) CAS#:69471-70-1
CAS#:69471-70-1
