4-Iodotoluene

4-Iodotoluene structure
|
Common Name | 4-Iodotoluene | ||
|---|---|---|---|---|
| CAS Number | 624-31-7 | Molecular Weight | 218.035 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 211.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C7H7I | Melting Point | 33-35 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 90.0±0.0 °C | |
| Name | 1-iodo-4-methylbenzene |
|---|---|
| Synonym | More Synonyms |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 211.5±0.0 °C at 760 mmHg |
| Melting Point | 33-35 °C(lit.) |
| Molecular Formula | C7H7I |
| Molecular Weight | 218.035 |
| Flash Point | 90.0±0.0 °C |
| Exact Mass | 217.959229 |
| LogP | 3.71 |
| Vapour Pressure | 0.3±0.4 mmHg at 25°C |
| Index of Refraction | 1.605 |
| InChIKey | UDHAWRUAECEBHC-UHFFFAOYSA-N |
| SMILES | Cc1ccc(I)cc1 |
| Water Solubility | insoluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29036990 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2903999090 |
|---|---|
| Summary | 2903999090 halogenated derivatives of aromatic hydrocarbons VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Rational strategy for shaped nanomaterial synthesis in reverse micelle reactors.
Nat. Commun. 5 , 3870, (2014) The shape-controlled synthesis of nanoparticles was established in single-phase solutions by controlling growth directions of crystalline facets on seed nanocrystals kinetically; however, it was diffi... |
|
|
Transition metal complexes bearing NHC ligands substituted with secondary polyfluoroalkyl groups.
Dalton Trans. 44 , 19663-73, (2015) Using three different approaches, racemic 1-(perfluoroalkyl)ethylamines were synthesized from perfluoroalkyl iodides or perfluoroalkanoic acids, and further transformed to the corresponding N,N'-disub... |
|
|
Cobalt-catalyzed aryl-sulfur bond formation.
Org. Lett. 8 , 5613, (2006) A new cobalt-catalyzed coupling of aryl halides with thiophenols and alkanethiols is reported. A variety of aryl sulfides can be prepared in excellent yields under mild reaction conditions using 1-2 m... |
| p-Methyliodobenzene |
| 4-IODO-TOLUENE |
| Toluene,p-iodo |
| 4-Iodotoluene |
| Benzene, 1-iodo-4-methyl- |
| 4-methyliodobenzene |
| 1-Methyl-4-iodobenzene |
| Benzene,1-iodo-4-methyl |
| 4-iodo-1-methylbenzene |
| p-Tolyl iodide |
| p-Iodotoluene |
| 4-methyl-1-iodobenzene |
| MFCD00001059 |
| 1-Iodo-4-methylbenzene |
| Toluene, p-iodo- |
| EINECS 210-841-7 |
| 4-methylphenyl iodide |
| para-methyl-iodo-benzene |
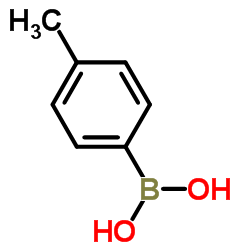 CAS#:5720-05-8
CAS#:5720-05-8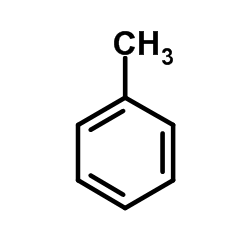 CAS#:108-88-3
CAS#:108-88-3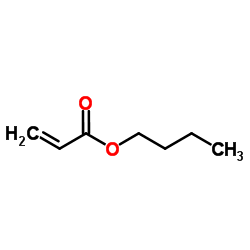 CAS#:141-32-2
CAS#:141-32-2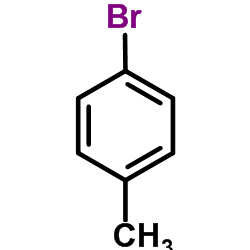 CAS#:106-38-7
CAS#:106-38-7 CAS#:106-43-4
CAS#:106-43-4 CAS#:106-49-0
CAS#:106-49-0 CAS#:99-94-5
CAS#:99-94-5 CAS#:3728-43-6
CAS#:3728-43-6![1-[[(4-METHYLPHENYL)SULPHONYL]OXY]-1,2-BENZIODOXOL-3(1H)-ONE Structure](https://image.chemsrc.com/caspic/412/159950-96-6.png) CAS#:159950-96-6
CAS#:159950-96-6 CAS#:18282-51-4
CAS#:18282-51-4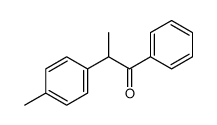 CAS#:107271-15-8
CAS#:107271-15-8 CAS#:106614-58-8
CAS#:106614-58-8 CAS#:10519-06-9
CAS#:10519-06-9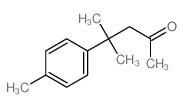 CAS#:10528-65-1
CAS#:10528-65-1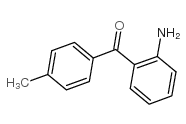 CAS#:36192-63-9
CAS#:36192-63-9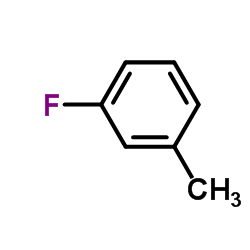 CAS#:352-70-5
CAS#:352-70-5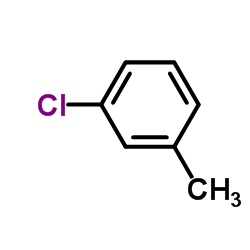 CAS#:108-41-8
CAS#:108-41-8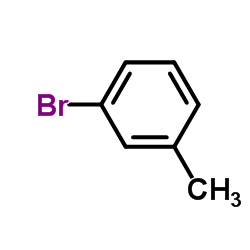 CAS#:591-17-3
CAS#:591-17-3
