FORMAMIDOXIME
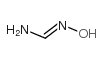
FORMAMIDOXIME structure
|
Common Name | FORMAMIDOXIME | ||
|---|---|---|---|---|
| CAS Number | 624-82-8 | Molecular Weight | 60.05530 | |
| Density | 1.29g/cm3 | Boiling Point | 224ºC at 760 mmHg | |
| Molecular Formula | CH4N2O | Melting Point | 112-115ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 89.3ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
| Name | formamidoxime |
|---|---|
| Synonym | More Synonyms |
| Density | 1.29g/cm3 |
|---|---|
| Boiling Point | 224ºC at 760 mmHg |
| Melting Point | 112-115ºC(lit.) |
| Molecular Formula | CH4N2O |
| Molecular Weight | 60.05530 |
| Flash Point | 89.3ºC |
| Exact Mass | 60.03240 |
| PSA | 58.61000 |
| LogP | 0.06290 |
| Index of Refraction | 1.471 |
| InChIKey | IONSZLINWCGRRI-UHFFFAOYSA-N |
| SMILES | NC=NO |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H351 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22-40 |
| Safety Phrases | 22-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LQ4632000 |
| HS Code | 2925290090 |
|
~% 
FORMAMIDOXIME CAS#:624-82-8 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 280, p. 325 |
|
~% 
FORMAMIDOXIME CAS#:624-82-8 |
| Literature: Liebigs Annalen der Chemie, , vol. 166, p. 295 - 320 |
|
~% 
FORMAMIDOXIME CAS#:624-82-8 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 166, p. 295 |
|
~% 
FORMAMIDOXIME CAS#:624-82-8 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 166, p. 295 |
|
~%
Detail
|
| Literature: Journal fuer Praktische Chemie (Leipzig), , vol. <2>105, p. 24 |
|
~%
Detail
|
| Literature: Journal fuer Praktische Chemie (Leipzig), , vol. <2>105, p. 24 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Antitumor activity of amidoximes (hydroxyurea analogs) in murine tumor systems.
Cancer Res. 38(5) , 1291-5, (1978) A series of amidoximes was prepared and evaluated for possible antitumor activity against L1210 leukemia. Three of the most active compounds in the L1210 system, formamidoxime, acetamidoxime, and 2-am... |
|
|
Nitric oxide synthesis by tracheal smooth muscle cells by a nitric oxide synthase-independent pathway.
Am. J. Physiol. 275(5 Pt 1) , L895-901, (1998) Nitric oxide (NO) is known to be synthesized from L-arginine in a reaction catalyzed by NO synthase. Liver cytochrome P-450 enzymes also catalyze the oxidative cleavage of C==N bonds of compounds cont... |
|
|
Effects of phenobarbital, beta-naphthoflavone, dexamethasone, and formamidoxime on the turnover of inducible microsomal proteins in cultured hepatocytes.
J. Biol. Chem. 256(24) , 13079-84, (1981) Microsomal proteins from cultured chick embryo hepatocytes were separated by polyacrylamide gel electrophoresis and their rate constants of degradation (Kd) were estimated using double isotope techniq... |
| EINECS 210-865-8 |
| carboxamidoxime |
| Aminoformaldehyde oxime |
| formamideoxime |
| N-hydroxycarboximidamide |
| n-hydroxy-methanimidamid |
| formamidoxim |
| n-hydroxymethanimidamide |
| isuretin |
| MFCD00008125 |
| isouretin |


 CAS#:64-18-6
CAS#:64-18-6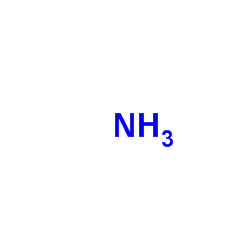 CAS#:7664-41-7
CAS#:7664-41-7 CAS#:591-07-1
CAS#:591-07-1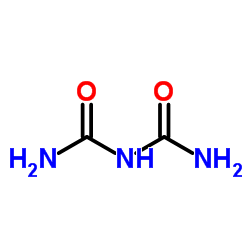 CAS#:108-19-0
CAS#:108-19-0 CAS#:463-79-6
CAS#:463-79-6 CAS#:92304-25-1
CAS#:92304-25-1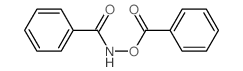 CAS#:959-32-0
CAS#:959-32-0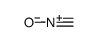 CAS#:51060-05-0
CAS#:51060-05-0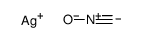 CAS#:5610-59-3
CAS#:5610-59-3