Tozasertib
Modify Date: 2025-08-21 17:17:42

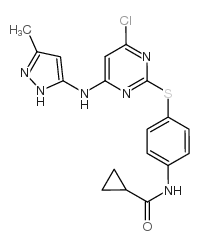
Tozasertib structure
|
Common Name | Tozasertib | ||
|---|---|---|---|---|
| CAS Number | 639089-54-6 | Molecular Weight | 464.586 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C23H28N8OS | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of TozasertibTozasertib is the inhibitor of Aurora A/B/C kinases with Kis of 0.6, 18, 4.6 nM, respectively. |
| Name | Tozasertib |
|---|---|
| Synonym | More Synonyms |
| Description | Tozasertib is the inhibitor of Aurora A/B/C kinases with Kis of 0.6, 18, 4.6 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Aurora A:0.6 nM (Ki) Aurora B:18 nM (Ki) Aurora C:4.6 nM (Ki) |
| In Vitro | Tozasertib induces similar cytotoxicity with IC50 of approximately 300 nM and exhibits an AUR B-like inhibitory phenotype of G2/M arrest, endoreduplication and apoptosis in BaF3 cells transfected with ABL or FLT-3 (mutant and wild type) kinases. Tozasertib prevents the CAL-62 proliferation in a time-dependent manner. Tozasertib treatment for 14 days significantly decreases the number and size of colonies by approximately 70% in the 8305C and 90% in the CAL-62, 8505C and BHT-101. Treatment of the different ATC cells with Tozasertib inhibits proliferation with the IC50 between 25 and 150 nM. The Tozasertib significantly impairs the ability of the different cell lines to form colonies in soft agar. Analysis of caspase-3 activity indicates that Tozasertib induces apoptosis in the different cell lines. CAL-62 cells exposed for 12 hours to Tozasertib show an accumulation of cells with ≥ 4N DNA content. Time-lapse analysis demonstrates that Tozasertib-treated CAL-62 cells exit metaphase without dividing. Moreover, histone H3 phosphorylation is abrogated following Tozasertib treatment[2]. Tozasertib has significant inhibitory activity against BCR-Abl bearing the T315I mutation in patient-derived samples[3]. |
| Kinase Assay | The consumption of ATP is coupled via the pyruvate kinase/lactic dehydrogenase enzyme pair to the oxidation of NADH, which can be monitored through the decrease in absorption at 340 nm. Reactions contains 100 mM Tris (pH 8), 10 mM MgCl2, 2.2 mM ATP, 1 mM phosphoenolpyruvate, 0.6 mg/mL NADH, 75 units/mL pyruvate kinase, 105 units/mL lactate dehydrogenase, and 0.5 mM substrate peptide (sequence: EAIYAAPFAKKK). Reactions (75 μL) are started by adding sufficient kinase to bring the reactions to 30 nM kinase concentration and the decrease in absorbance is monitored over 30 minutes at 30°C in a microtiter plate spectrophotometer. Inhibitory constants are obtained through addition of 3.75 μL Tozasertib in 100% DMSO or DMSO alone. Ki values are calculated as follows, Ki=IC50/(1+[S]/Kd), where [S]=[ATP]=2.2 mM, and Kd (of ATP to Abl)=70 μM. These values are calculated assuming a Kd (ATP) of 70 μM for wild type and H396P Abl kinase domain. |
| Cell Assay | The CAL-62 cells are cultured in the absence (dimethyl sulfoxide, DMSO) or the presence of 500 nM Tozasertib for different periods of time (1-5 days). The dose-dependent effects of Tozasertib on cell proliferation are evaluated by treating the different ATC cells for 4 days with different concentrations of the Aurora inhibitor (5-500 nM). The cells are pulse labeled with 30 mM BrdU for 2 hours before the end of the incubation time. The BrdU incorporation is analyzed by means of a colorimetric immunoassay using the cell proliferation ELISA kit. The results from Tozasertib-treated cells are compared with those observed in control cells and expressed as a fold of variation versus control. |
| Animal Admin | For the HL-60 study, female athymic NCr-nu mice are inoculated subcutaneously with 107 HL-60(TB) leukemia cells into the right axillary area. Treatment is administered i.p. b.i.d. after tumors reached 150−200 mm3. Tozasertib is prepared in a vehicle of 50% PEG 300 in 50 mM phosphate buffer. Cisplatin, formulated in saline, is administered i.p. q.4.d. for a total of three injections, at a dose of 5.4 mg/kg. For the MIA PaCa-2 studies, female MF1 nude mice are inoculated with 107 MIA PaCa-2 cells into the dorsal flank. Treatment is administered i.p. b.i.d. after tumors reach 175 mm3. Tozasertib is prepared in a vehicle of 50% PEG 300 in 50 mM phosphate buffer. 5-fluorouracil, formulated in saline, is administered i.v. q.4.d. at a dose of 50 mg/kg. For the HCT116 study, female Hsd RH rnu/nu rats are inoculated with 107 HCT116 cells into the right flank. Treatment is administered once the tumors reached 700−950 mm3. Tozasertib is administered continuously through an indwelling femoral catheter, followed by a saline infusion for 4 d before repeating the dose cycle. For all studies, tumor volume is determined by caliper measurements three times a week. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C23H28N8OS |
| Molecular Weight | 464.586 |
| Exact Mass | 464.210663 |
| PSA | 127.37000 |
| LogP | 1.18 |
| Index of Refraction | 1.708 |
| InChIKey | GCIKSSRWRFVXBI-UHFFFAOYSA-N |
| SMILES | Cc1cc(Nc2cc(N3CCN(C)CC3)nc(Sc3ccc(NC(=O)C4CC4)cc3)n2)n[nH]1 |
| Storage condition | -20°C |
|
~66% 
Tozasertib CAS#:639089-54-6 |
| Literature: MERCK and CO., INC. Patent: WO2008/13807 A2, 2008 ; Location in patent: Page/Page column 7 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| N-[4-({4-(4-Methylpiperazin-1-yl)-6-[(3-methyl-1H-pyrazol-5-yl)amino]pyrimidin-2-yl}sulfanyl)phenyl]cyclopropanecarboxamide |
| VX-680 |
| MK-0457 |
| Tozasertib Free Base |
| Cyclopropanecarboxamide, N-[4-[[4-(4-methyl-1-piperazinyl)-6-[(5-methyl-1H-pyrazol-3-yl)amino]-2-pyrimidinyl]thio]phenyl]- |
| Tozasertib |
| N-[4-({4-(4-Methyl-1-piperazinyl)-6-[(5-methyl-1H-pyrazol-3-yl)amino]-2-pyrimidinyl}sulfanyl)phenyl]cyclopropanecarboxamide |