methylpiperazine
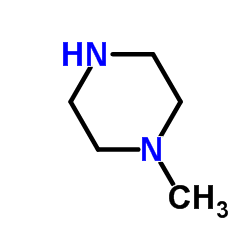
methylpiperazine structure
|
Common Name | methylpiperazine | ||
|---|---|---|---|---|
| CAS Number | 109-01-3 | Molecular Weight | 100.162 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 138.0±8.0 °C at 760 mmHg | |
| Molecular Formula | C5H12N2 | Melting Point | -6 °C | |
| MSDS | Chinese USA | Flash Point | 42.2±0.0 °C | |
| Symbol |



GHS02, GHS05, GHS06 |
Signal Word | Danger | |
| Name | 1-Methylpiperazine |
|---|---|
| Synonym | More Synonyms |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 138.0±8.0 °C at 760 mmHg |
| Melting Point | -6 °C |
| Molecular Formula | C5H12N2 |
| Molecular Weight | 100.162 |
| Flash Point | 42.2±0.0 °C |
| Exact Mass | 100.100044 |
| PSA | 15.27000 |
| LogP | -0.18 |
| Vapour density | 3.5 (vs air) |
| Vapour Pressure | 6.9±0.3 mmHg at 25°C |
| Index of Refraction | 1.442 |
| InChIKey | PVOAHINGSUIXLS-UHFFFAOYSA-N |
| SMILES | CN1CCNCC1 |
| Storage condition | Store under Nitrogen |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H226-H312-H314-H331 |
| Precautionary Statements | P261-P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive |
| Risk Phrases | R10;R21;R34 |
| Safety Phrases | S16-S26-S36/37/39-S45 |
| RIDADR | UN 2734 8/PG 2 |
| WGK Germany | 2 |
| RTECS | TM1225000 |
| Packaging Group | III |
| Hazard Class | 3 |
| HS Code | 2933599090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and antitumor activities of some new N1-(flavon-6-yl)amidrazone derivatives.
Arch. Pharm. (Weinheim) 347(6) , 415-22, (2014) A new series of N1-(flavon-6-yl)amidrazones were synthesized by reacting the hydrazonoyl chloride derived from 6-aminoflavone with the appropriate sec-cyclic amines. The antitumor activities of these ... |
|
|
Structure-activity relationships of a novel pyranopyridine series of Gram-negative bacterial efflux pump inhibitors.
Bioorg. Med. Chem. 23(9) , 2024-34, (2015) Recently we described a novel pyranopyridine inhibitor (MBX2319) of RND-type efflux pumps of the Enterobacteriaceae. MBX2319 (3,3-dimethyl-5-cyano-8-morpholino-6-(phenethylthio)-3,4-dihydro-1H-pyrano[... |
|
|
Synthesis of 1H-1,2,3-triazole linked aryl(arylamidomethyl) - dihydrofurocoumarin hybrids and analysis of their cytotoxicity.
Eur. J. Med. Chem. 100 , 119-28, (2015) A series of 2-(4-R-triazolyl)substituted 3-oxo-2,3-dihydrofurocoumarins have been synthesized by a regioselective cycloaddition of 2-azidooreoselone 1 or 2-azido-9-[(4-methylpiperazin-1-yl)methyl]oreo... |
| Piperazine, 1-methyl- |
| methyl piperazine |
| 4-Methylpiperazine |
| Cyclizine Related Compound A |
| N-METHYL-PIPERAZINE |
| EINECS 203-639-5 |
| N-Methylpiperazine |
| PIPERAZINE,1-METHYL |
| N'-Methylpiperazine |
| 1-Methyl-piperazine |
| 1-Methylpiperazine |
| MFCD00005966 |
| methylpiperazine |
| N-METHYL PIPERAZINE |
 CAS#:111-42-2
CAS#:111-42-2 CAS#:74-89-5
CAS#:74-89-5 CAS#:325812-49-5
CAS#:325812-49-5 CAS#:110-85-0
CAS#:110-85-0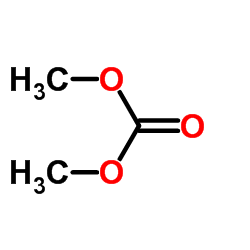 CAS#:616-38-6
CAS#:616-38-6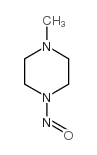 CAS#:16339-07-4
CAS#:16339-07-4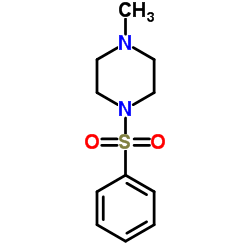 CAS#:66739-87-5
CAS#:66739-87-5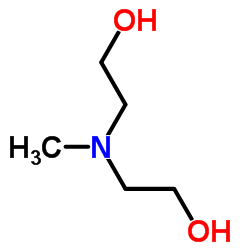 CAS#:105-59-9
CAS#:105-59-9 CAS#:62226-74-8
CAS#:62226-74-8 CAS#:105396-59-6
CAS#:105396-59-6 CAS#:100491-52-9
CAS#:100491-52-9 CAS#:108372-23-2
CAS#:108372-23-2![[4-methoxy-2-[4-(4-methylpiperazin-1-yl)-4-oxo-1-phenylbutyl]phenyl] acetate structure](https://image.chemsrc.com/caspic/328/104712-26-7.png) CAS#:104712-26-7
CAS#:104712-26-7![2-Acetyl-10-[3-(4-methylpiperazino)propyl]-10H-phenothiazine structure](https://image.chemsrc.com/caspic/095/1053-74-3.png) CAS#:1053-74-3
CAS#:1053-74-3 CAS#:1059705-52-0
CAS#:1059705-52-0 CAS#:110358-10-6
CAS#:110358-10-6 CAS#:1093065-11-2
CAS#:1093065-11-2 CAS#:56323-03-6
CAS#:56323-03-6 CAS#:55480-45-0
CAS#:55480-45-0
