Oltipraz
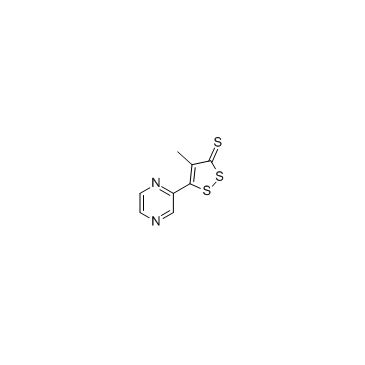
Oltipraz structure
|
Common Name | Oltipraz | ||
|---|---|---|---|---|
| CAS Number | 64224-21-1 | Molecular Weight | 226.342 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 408.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C8H6N2S3 | Melting Point | 165-166ºC | |
| MSDS | Chinese USA | Flash Point | 200.6±31.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of OltiprazOltipraz has an inhibitory effect on HIF-1α activation by insulin in a time-dependent manner, completely abrogating HIF-1α induction at ≥10 μM concentrations, the IC50 of Oltipraz for HIF-1α inhibition is 10 μM.IC50 value: 10 μMTarget: HIF-1αin vitro: Oltipraz inhibits HIF-1α activity and HIF-1α-dependent tumor growth, which may result from a decrease in HIF-1α stability through S6K1 inhibition in combination with an H2O2-scavenging effect. Oltipraz treatment also inhibits HIF-1α activation stimulated by either hypoxia or CoCl2. Oltipraz is a cancer chemopreventive agent and has an inhibitory effect on angiogenesis and tumor growth. [1] Oltipraz is also a competitive inhibitor of this cytochrome P450, with an apparent Ki of 10 μM. [2]in vivo: In wild-type mice, hepatic levels of mRNA for all of the genes analyzed were significantly increased after Oltipraz treatment, with the highest increase (treated/control) for NQO1 mRNA levels (7.6-fold). The Northern blot analyses demonstrated that the observed increases in GST and NQO1 activities by Oltipraz in wild-type mice were preceded by significant elevations in RNA expression. Interestingly, mRNA levels of Nrf2 itself were increased more than 3-fold by Oltipraz treatment. [2] |
| Name | oltipraz |
|---|---|
| Synonym | More Synonyms |
| Description | Oltipraz has an inhibitory effect on HIF-1α activation by insulin in a time-dependent manner, completely abrogating HIF-1α induction at ≥10 μM concentrations, the IC50 of Oltipraz for HIF-1α inhibition is 10 μM.IC50 value: 10 μMTarget: HIF-1αin vitro: Oltipraz inhibits HIF-1α activity and HIF-1α-dependent tumor growth, which may result from a decrease in HIF-1α stability through S6K1 inhibition in combination with an H2O2-scavenging effect. Oltipraz treatment also inhibits HIF-1α activation stimulated by either hypoxia or CoCl2. Oltipraz is a cancer chemopreventive agent and has an inhibitory effect on angiogenesis and tumor growth. [1] Oltipraz is also a competitive inhibitor of this cytochrome P450, with an apparent Ki of 10 μM. [2]in vivo: In wild-type mice, hepatic levels of mRNA for all of the genes analyzed were significantly increased after Oltipraz treatment, with the highest increase (treated/control) for NQO1 mRNA levels (7.6-fold). The Northern blot analyses demonstrated that the observed increases in GST and NQO1 activities by Oltipraz in wild-type mice were preceded by significant elevations in RNA expression. Interestingly, mRNA levels of Nrf2 itself were increased more than 3-fold by Oltipraz treatment. [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 408.1±55.0 °C at 760 mmHg |
| Melting Point | 165-166ºC |
| Molecular Formula | C8H6N2S3 |
| Molecular Weight | 226.342 |
| Flash Point | 200.6±31.5 °C |
| Exact Mass | 225.969315 |
| PSA | 114.35000 |
| LogP | 1.92 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.760 |
| InChIKey | CKNAQFVBEHDJQV-UHFFFAOYSA-N |
| SMILES | Cc1c(-c2cnccn2)ssc1=S |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | NONH for all modes of transport |
| RTECS | JP1293000 |
| HS Code | 2933990090 |
|
~14% 
Oltipraz CAS#:64224-21-1 |
| Literature: CJ CORPORATION Patent: WO2004/48369 A1, 2004 ; Location in patent: Page 9-10 ; |
|
~% 
Oltipraz CAS#:64224-21-1 |
| Literature: US2004/53989 A1, ; |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice.
Diabetes 63(10) , 3483-96, (2014) This study investigated the impact of catalase (Cat) overexpression in renal proximal tubule cells (RPTCs) on nuclear factor erythroid 2-related factor 2 (Nrf2) stimulation of angiotensinogen (Agt) ge... |
|
|
Antioxidant and mitochondrial protective effects of oxidized metabolites of oltipraz.
Expert Opin. Drug Metab. Toxicol. 6(2) , 213-24, (2010) Comprehensive studies indicate that oltipraz exerts cancer chemopreventive effects. Oltipraz has other therapeutic potentials, which include anti-fibrotic effect, inhibition of insulin resistance, mit... |
|
|
Inhibition of colon carcinogenesis by post-initiation induction of NQO1 in Sprague-Dawley rats.
Oncol. Rep. 21(6) , 1559-65, (2009) Inducers of phase II detoxifying enzymes have been studied as chemopreventive agents for a variety of cancers. Phase II detoxifying enzymes may play a significant role in preventing carcinogen-induced... |
| Oltipraz |
| 4-methyl-5-pyrazin-2-yldithiole-3-thione |
| RP-35,972 |
| 4-Methyl-5-(pyrazin-2-yl)-3H-1,2-dithiole-3-thione |
| 4-Methyl-5-(2-pyrazinyl)-3H-1,2-dithiole-3-thione |
| 4-Methyl-5-pyrazinyl-3H-1,2-dithiole-3-thione |
| 3H-1,2-DITHIOLE-3-THIONE,4-METHYL-5-PYRAZINYL |
| 4-Methyl-5-pyrazin-2-yl-1,2-dithiole-3-thione |
| 3H-1,2-Dithiole-3-thione, 4-methyl-5-(2-pyrazinyl)- |

 CAS#:84201-40-1
CAS#:84201-40-1![6-(ethyldisulfanyl)-7-methyl-8-(methylthio)pyrrolo[1,2-a]pyrazine structure](https://image.chemsrc.com/caspic/030/114969-39-0.png) CAS#:114969-39-0
CAS#:114969-39-0 CAS#:87578-53-8
CAS#:87578-53-8![6,8-dimethylthio-7-formylpyrrolo [1,2-a] pyrazine structure](https://image.chemsrc.com/caspic/443/103428-51-9.png) CAS#:103428-51-9
CAS#:103428-51-9
