Nicorandil
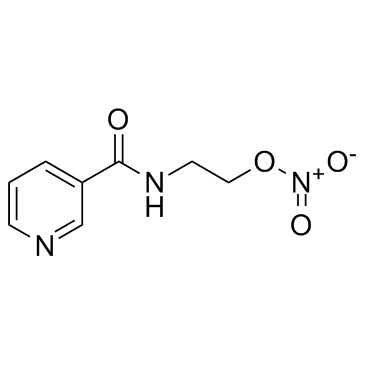
Nicorandil structure
|
Common Name | Nicorandil | ||
|---|---|---|---|---|
| CAS Number | 65141-46-0 | Molecular Weight | 211.175 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 456.7±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H9N3O4 | Melting Point | 92ºC | |
| MSDS | Chinese USA | Flash Point | 230.0±23.2 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of NicorandilNicorandil is potassium channel activator.Target: Potassium ChannelNicorandil is a vasodilatory drug used to treat angina. Nicorandil stimulates guanylate cyclase to increase formation of cyclic GMP (cGMP). cGMP activates protein kinase G (PKG) which phosphorylates and inhibits GTPase RhoA and decreases Rho-kinase activity. Reduced Rho-kinase activity permits an increase in myosin phosphatase activity, decreasing the calcium sensitivity of the smooth muscle. PKG also activates the sarcolemma calcium pump to remove activating calcium. PKG acts on K+ channels to promote K+ efflux and the ensuing hyperpolarization inhibits voltage-gated calcium channels. Overall, this leads to relaxation of the smooth muscle and coronary vasodilation [1, 2]. |
| Name | nicorandil |
|---|---|
| Synonym | More Synonyms |
| Description | Nicorandil is potassium channel activator.Target: Potassium ChannelNicorandil is a vasodilatory drug used to treat angina. Nicorandil stimulates guanylate cyclase to increase formation of cyclic GMP (cGMP). cGMP activates protein kinase G (PKG) which phosphorylates and inhibits GTPase RhoA and decreases Rho-kinase activity. Reduced Rho-kinase activity permits an increase in myosin phosphatase activity, decreasing the calcium sensitivity of the smooth muscle. PKG also activates the sarcolemma calcium pump to remove activating calcium. PKG acts on K+ channels to promote K+ efflux and the ensuing hyperpolarization inhibits voltage-gated calcium channels. Overall, this leads to relaxation of the smooth muscle and coronary vasodilation [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.7±25.0 °C at 760 mmHg |
| Melting Point | 92ºC |
| Molecular Formula | C8H9N3O4 |
| Molecular Weight | 211.175 |
| Flash Point | 230.0±23.2 °C |
| Exact Mass | 211.059311 |
| PSA | 97.04000 |
| LogP | 0.72 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.548 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318 |
| Precautionary Statements | P280-P301 + P312 + P330-P305 + P351 + P338 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22-41 |
| Safety Phrases | 26-39 |
| RIDADR | NONH for all modes of transport |
| RTECS | US4667600 |
| HS Code | 2933399090 |
|
~% 
Nicorandil CAS#:65141-46-0 |
| Literature: WO2010/673 A1, ; Page/Page column 11-12 ; |
|
~% 
Nicorandil CAS#:65141-46-0 |
| Literature: WO2012/89769 A1, ; Page/Page column 9-10 ; |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Leg ulcers associated with nicorandil are possibly underdiagnosed.
Clin. Exp. Dermatol. 38(2) , 193-4, (2013)
|
|
|
Treating stable angina - is there a NICE way towards an international consensus?
Int. J. Clin. Pract. 66(7) , 614-8, (2012)
|
|
|
Peristomal ulceration caused by nicorandil.
Clin. Exp. Dermatol. 34(1) , 87-8, (2009)
|
| 3-Pyridinecarboxamide, N-[2-(nitrooxy)ethyl]- |
| Nitorubin |
| Perisalol |
| 2-(pyridine-3-carbonylamino)ethyl nitrate |
| 2-[(Pyridin-3-ylcarbonyl)amino]ethylnitrat |
| Nicorandil |
| Ikorel |
| SG-75 |
| Dancor |
| EINECS 265-514-1 |
| Nikoran |
| Nicorandilum |
| 2-[(Pyridin-3-ylcarbonyl)amino]ethyl nitrate |
| N-(2-hydroxyethyl)nicotinamide nitrate ester |
| 2-[(3-Pyridinylcarbonyl)amino]ethyl nitrate |
| Adancor |
| N-[2-(nitroxy)ethyl]-3-pyridine carboxamide |
| Sigmart |
| 2-Nicotinamidoethyl nitrate |


 CAS#:40055-37-6
CAS#:40055-37-6