(S)-(+)-N,N-DIMETHYL-3-(1-NAPHTHALENYLOXY)-3-(2-THIENYL)PROPANAMINE
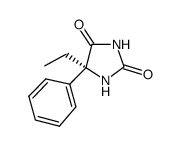
(S)-(+)-N,N-DIMETHYL-3-(1-NAPHTHALENYLOXY)-3-(2-THIENYL)PROPANAMINE structure
|
Common Name | (S)-(+)-N,N-DIMETHYL-3-(1-NAPHTHALENYLOXY)-3-(2-THIENYL)PROPANAMINE | ||
|---|---|---|---|---|
| CAS Number | 65567-34-2 | Molecular Weight | 204.22500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H12N2O2 | Melting Point | 195-200ºC | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | (s)-(+)-nirvanol |
|---|
| Melting Point | 195-200ºC |
|---|---|
| Molecular Formula | C11H12N2O2 |
| Molecular Weight | 204.22500 |
| Exact Mass | 204.09000 |
| PSA | 58.20000 |
| LogP | 1.78890 |
| Appearance of Characters | off-white |
| InChIKey | UDTWZFJEMMUFLC-NSHDSACASA-N |
| SMILES | CCC1(c2ccccc2)NC(=O)NC1=O |
| Storage condition | 2-8°C |
| Water Solubility | DMF: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn,Xi |
| Risk Phrases | 22-36/37/38-20/21/22 |
| Safety Phrases | 26-37/39-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | MU2452000 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Pharmacodynamics of cytochrome P450 2B induction by phenobarbital, 5-ethyl-5-phenylhydantoin, and 5-ethyl-5-phenyloxazolidinedione in the male rat liver or in cultured rat hepatocytes.
Chem. Res. Toxicol. 6(2) , 188-96, (1993) The pharmacodynamics of rat hepatic cytochrome P450 2B (P450 2B) induction by phenobarbital (PB) and two structural congeners, dl-5-ethyl-5-phenylhydantoin (EPH) and dl-5-ethyl-5-phenyloxazolidinedion... |
|
|
A markedly diminished pleiotropic response to phenobarbital and structurally-related xenobiotics in Zucker rats in comparison with F344/NCr or DA rats.
Biochem. Pharmacol. 43(5) , 1079-87, (1992) Phenobarbital (PB) and certain structurally-related compounds induce a variety of hepatic drug-metabolizing enzymes in many strains of rats. Thus, following administration of PB (300, 500 ppm), barbit... |
|
|
Active-site characteristics of CYP2C19 and CYP2C9 probed with hydantoin and barbiturate inhibitors.
Arch. Biochem. Biophys. 429(1) , 1-15, (2004) Three series of N-3 alkyl substituted phenytoin, nirvanol, and barbiturate derivatives were synthesized and their inhibitor potencies were tested against recombinant CYP2C19 and CYP2C9 to probe the in... |
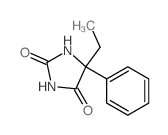 CAS#:2216-93-5
CAS#:2216-93-5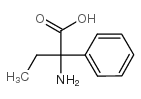 CAS#:5438-07-3
CAS#:5438-07-3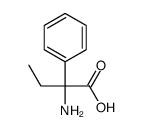 CAS#:52247-77-5
CAS#:52247-77-5 CAS#:33875-36-4
CAS#:33875-36-4 CAS#:57-13-6
CAS#:57-13-6 CAS#:70989-04-7
CAS#:70989-04-7