(S)-Mephenytoin
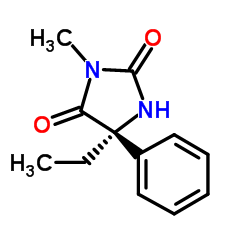
(S)-Mephenytoin structure
|
Common Name | (S)-Mephenytoin | ||
|---|---|---|---|---|
| CAS Number | 70989-04-7 | Molecular Weight | 218.252 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C12H14N2O2 | Melting Point | 135-138ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (S)-Mephenytoin(S)-Mephenytoin ((+)-Mephenytoin) is an anticonvulsive agent. (S)-Mephenytoin is a substrate of the cytochrome P450 (CYP) isoform CYP2C19. (S)-Mephenytoin can be used for the analysis of cytochrome P450 metabolism[1][2]. |
| Name | (s)-mephenytoin |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-Mephenytoin ((+)-Mephenytoin) is an anticonvulsive agent. (S)-Mephenytoin is a substrate of the cytochrome P450 (CYP) isoform CYP2C19. (S)-Mephenytoin can be used for the analysis of cytochrome P450 metabolism[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | In the presence of cytochrome b5, the Km for S-mephenytoin is 1.25 mM with all five purified cytochrome P-450s preparations[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 135-138ºC |
| Molecular Formula | C12H14N2O2 |
| Molecular Weight | 218.252 |
| Exact Mass | 218.105530 |
| PSA | 49.41000 |
| LogP | 1.40 |
| Index of Refraction | 1.541 |
| InChIKey | GMHKMTDVRCWUDX-LBPRGKRZSA-N |
| SMILES | CCC1(c2ccccc2)NC(=O)N(C)C1=O |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22-36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine.
Malaria Journal 11 , 259, (2012) The 8-aminoquinoline (8AQ) drug primaquine (PQ) is currently the only approved drug effective against the persistent liver stage of the hypnozoite forming strains Plasmodium vivax and Plasmodium ovale... |
|
|
Trade herbal products and induction of CYP2C19 and CYP2E1 in cultured human hepatocytes.
Basic Clin Pharmacol Toxicol. 105(1) , 58-63, (2009) The aim of this study was to evaluate in vitro the dose-dependent induction potential of six commonly used trade herbal products on CYP2C19 and CYP2E1 metabolic activities in cultured human hepatocyte... |
| S(+)-5-Ethyl-3-methyl-5-phenylhydantoin |
| S(+)-5-Ethyl-1-methyl-5-phenylbarbituric acid |
| S-mephenytoin |
| (+)-Mephobarbitol |
| 5-ethyl-3-methyl-5-phenylimidazolidine-2,4-dione |
| 5-éthyl-3-méthyl-5-phénylimidazolidine-2,4-dione |
| 5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione |
| (S)-Mephenytoin |
| mephenytoin |
| (5S)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione |
| [14C]-(+)-Mephenytoin |
| UNII:R420KW629U |
| 5-Ethyl-3-methyl-5-phenylimidazolidin-2,4-dion |
| 2,4,6(1H,3H,5H)-Pyrimidinetrione,5-ethyl-1-methyl-5-phenyl-,(S) |
| (+)-Mephobarbital |
| Gerot-Epilan |
| (5R)-mephobarbital |
| (S)-5-Ethyl-1-methyl-5-phenyl-2,4,6(1H,3H,5H)-pyrimidinetrione |
| 2,4-Imidazolidinedione, 5-ethyl-3-methyl-5-phenyl- |
| (S)-Methylphenobarbital |
| UNII-5NC67NU76B component ALARQZQTBTVLJV-ZDUSSCGKSA-N |
| 2,4-Imidazolidinedione, 5-ethyl-3-methyl-5-phenyl-, (5S)- |
 CAS#:65567-34-2
CAS#:65567-34-2