Benzyl 4-bromophenyl ether
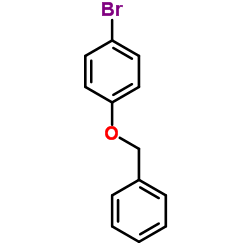
Benzyl 4-bromophenyl ether structure
|
Common Name | Benzyl 4-bromophenyl ether | ||
|---|---|---|---|---|
| CAS Number | 6793-92-6 | Molecular Weight | 263.130 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 340.6±17.0 °C at 760 mmHg | |
| Molecular Formula | C13H11BrO | Melting Point | 60-63 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 140.0±7.0 °C | |
| Name | 1-Benzyloxy-4-bromobenzene |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 340.6±17.0 °C at 760 mmHg |
| Melting Point | 60-63 °C(lit.) |
| Molecular Formula | C13H11BrO |
| Molecular Weight | 263.130 |
| Flash Point | 140.0±7.0 °C |
| Exact Mass | 261.999329 |
| PSA | 9.23000 |
| LogP | 4.82 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.600 |
| InChIKey | OUQSGILAXUXMGI-UHFFFAOYSA-N |
| SMILES | Brc1ccc(OCc2ccccc2)cc1 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2909309090 |
|---|---|
| Summary | 2909309090 other aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Synthesis and preliminary biological evaluation of new carbon-11 labeled tetrahydroisoquinoline derivatives as SERM radioligands for PET imaging of ER expression in breast cancer.
Eur. J. Med. Chem. 43 , 2211-9, (2008) The estrogen receptors (ERs) are attractive targets in the treatment of breast cancer and the development of receptor-based breast cancer imaging agents for diagnostic use in biomedical imaging techni... |
|
|
2-Arylmethyl-1,4-benzoquinones. II. Novel inhibitors of platelet aggregation: synthesis and pharmacological evaluation.
Chem. Pharm. Bull. 45(4) , 668-74, (1997) Two new series of 2-arylmethyl-1,4-benzoquinones (2 and 3) were synthesized for evaluation of their pharmacological activities. These compounds showed significant inhibition of platelet aggregation an... |
|
|
Aromatase and dual aromatase-steroid sulfatase inhibitors from the letrozole and vorozole templates.
ChemMedChem 6(8) , 1423-38, (2011) Concurrent inhibition of aromatase and steroid sulfatase (STS) may provide a more effective treatment for hormone-dependent breast cancer than monotherapy against individual enzymes, and several dual ... |
| Benzene, 1-bromo-4-(phenylmethoxy)- |
| 1-bromo-4-phenylmethoxybenzene |
| 1-(Benzyloxy)-4-bromobenzene |
| Benzyl 4-bromophenyl ether |
| Benzyl p-bromophenyl ether |
| MFCD00028016 |
 CAS#:106-41-2
CAS#:106-41-2 CAS#:100-39-0
CAS#:100-39-0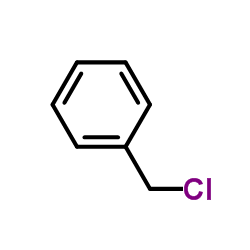 CAS#:100-44-7
CAS#:100-44-7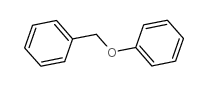 CAS#:946-80-5
CAS#:946-80-5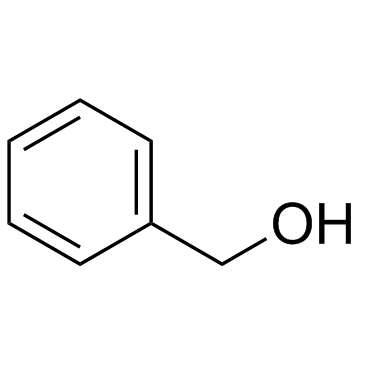 CAS#:100-51-6
CAS#:100-51-6 CAS#:836-43-1
CAS#:836-43-1 CAS#:87100-28-5
CAS#:87100-28-5 CAS#:66107-30-0
CAS#:66107-30-0 CAS#:67963-68-2
CAS#:67963-68-2 CAS#:17878-44-3
CAS#:17878-44-3 CAS#:606-87-1
CAS#:606-87-1 CAS#:142001-84-1
CAS#:142001-84-1 CAS#:19578-80-4
CAS#:19578-80-4 CAS#:6373-46-2
CAS#:6373-46-2 CAS#:74853-08-0
CAS#:74853-08-0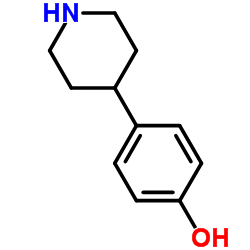 CAS#:62614-84-0
CAS#:62614-84-0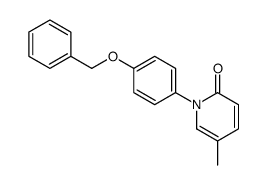 CAS#:1076199-02-4
CAS#:1076199-02-4
