2-(4-Methoxyphenyl)ethanol
Modify Date: 2025-08-25 08:09:20
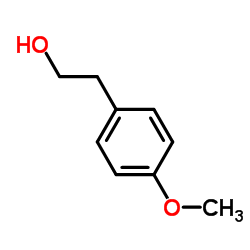
2-(4-Methoxyphenyl)ethanol structure
|
Common Name | 2-(4-Methoxyphenyl)ethanol | ||
|---|---|---|---|---|
| CAS Number | 702-23-8 | Molecular Weight | 152.190 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 257.5±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H12O2 | Melting Point | 26-28 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 110.2±14.6 °C | |
Use of 2-(4-Methoxyphenyl)ethanol4-Methoxyphenethyl alcohol, an aromatic alcohol, is the major component in the anise-like odour produced by A. albispathus Hett. 4-Methoxyphenethyl alcohol can inhibits the protein, RNA and DNA synthesis in Escherichia coli[1]. |
| Name | 4-Methoxyphenethyl Alcohol |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Methoxyphenethyl alcohol, an aromatic alcohol, is the major component in the anise-like odour produced by A. albispathus Hett. 4-Methoxyphenethyl alcohol can inhibits the protein, RNA and DNA synthesis in Escherichia coli[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 257.5±15.0 °C at 760 mmHg |
| Melting Point | 26-28 °C(lit.) |
| Molecular Formula | C9H12O2 |
| Molecular Weight | 152.190 |
| Flash Point | 110.2±14.6 °C |
| Exact Mass | 152.083725 |
| PSA | 29.46000 |
| LogP | 1.27 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.524 |
| InChIKey | AUWDOZOUJWEPBA-UHFFFAOYSA-N |
| SMILES | COc1ccc(CCO)cc1 |
| Storage condition | 2-8°C |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
|
An asymmetric catalytic carbon? carbon bond formation in a fluorous biphasic system based on perfluoroalkyl-BINOL. Tian Y and Chan KS.
Tetrahedron Lett. 41(45) , 8813-8816, (2000)
|
|
|
Biocatalytic anti-Prelog stereoselective reduction of 4'-methoxyacetophenone to (R)-1-(4-methoxyphenyl) ethanol with immobilized Trigonopsis variabilis AS2. 1611 cells using an ionic liquid-containing medium. Lou W-Y, et al.
Green Chem. 11(9) , 1377-1384, (2009)
|
|
|
Synthesis of 5-(ω-sulfhydrylalkyl) salicylaldehydes as precursors for the preparation of alkanethiol-modified metal salens. Ji C and Peters DG
Tetrahedron Lett. 42(35) , 6065-6067, (2001)
|
| MFCD00002900 |
| EINECS 211-866-6 |
| 2-(4-Methoxyphenyl)ethanol |
| 2-(p-Methoxyphenyl)ethyl alcohol |
| Benzeneethanol, 4-methoxy- |
 CAS#:104-01-8
CAS#:104-01-8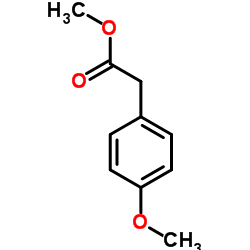 CAS#:23786-14-3
CAS#:23786-14-3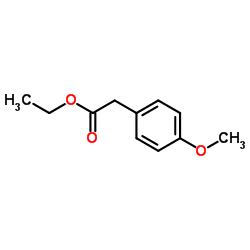 CAS#:14062-18-1
CAS#:14062-18-1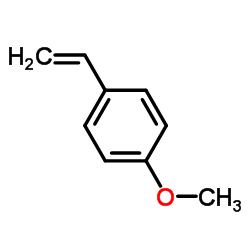 CAS#:637-69-4
CAS#:637-69-4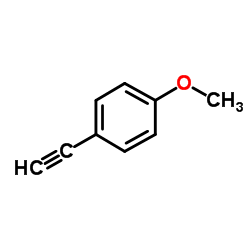 CAS#:768-60-5
CAS#:768-60-5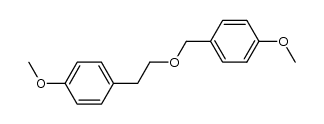 CAS#:156147-56-7
CAS#:156147-56-7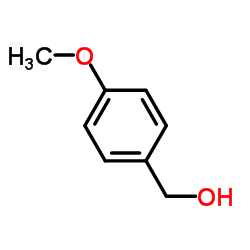 CAS#:105-13-5
CAS#:105-13-5 CAS#:4091-50-3
CAS#:4091-50-3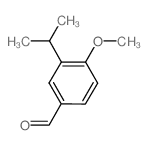 CAS#:31825-29-3
CAS#:31825-29-3 CAS#:4521-28-2
CAS#:4521-28-2 CAS#:100-09-4
CAS#:100-09-4 CAS#:5703-26-4
CAS#:5703-26-4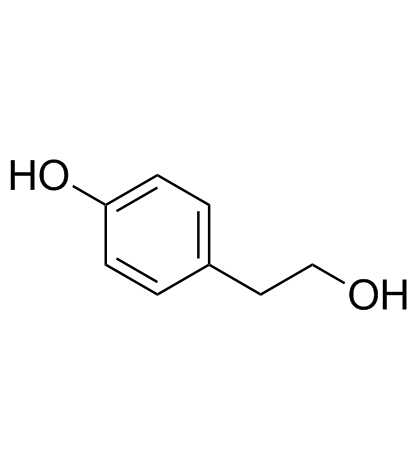 CAS#:501-94-0
CAS#:501-94-0 CAS#:18638-97-6
CAS#:18638-97-6 CAS#:22532-51-0
CAS#:22532-51-0
