Mevastatin
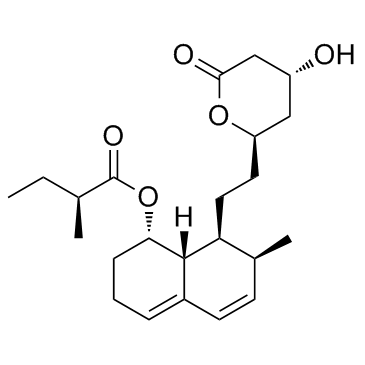
Mevastatin structure
|
Common Name | Mevastatin | ||
|---|---|---|---|---|
| CAS Number | 73573-88-3 | Molecular Weight | 390.513 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 555.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C23H34O5 | Melting Point | 151-153 °C | |
| MSDS | Chinese USA | Flash Point | 186.5±23.6 °C | |
Use of MevastatinMevastatin (Compactin; ML236B) inhibits HMGCR (HMG-CoA reductase) (Ki for acid form is 1 nM) which in turn inhibits isoprenoid biosynthesis and therefore blocks protein isoprenylation and reduces plasma cholesterol levels in humans. IC50 value: 1 nM (Ki)Target: HMGCRMevastatin induces apoptosis, arrests cancer cells in G1 phase and downregulates cdk 2, 4, and 6, cyclin D1 and E1, p21 and p27. Mevastatin suppresses TNF-induced NF-κB activation (IC50 = ~17 uM), which potentiates apoptosis in human myeloid leukemia cells and thus, may be useful in treating cancer. |
| Name | mevastatin |
|---|---|
| Synonym | More Synonyms |
| Description | Mevastatin (Compactin; ML236B) inhibits HMGCR (HMG-CoA reductase) (Ki for acid form is 1 nM) which in turn inhibits isoprenoid biosynthesis and therefore blocks protein isoprenylation and reduces plasma cholesterol levels in humans. IC50 value: 1 nM (Ki)Target: HMGCRMevastatin induces apoptosis, arrests cancer cells in G1 phase and downregulates cdk 2, 4, and 6, cyclin D1 and E1, p21 and p27. Mevastatin suppresses TNF-induced NF-κB activation (IC50 = ~17 uM), which potentiates apoptosis in human myeloid leukemia cells and thus, may be useful in treating cancer. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 555.0±50.0 °C at 760 mmHg |
| Melting Point | 151-153 °C |
| Molecular Formula | C23H34O5 |
| Molecular Weight | 390.513 |
| Flash Point | 186.5±23.6 °C |
| Exact Mass | 390.240631 |
| PSA | 72.83000 |
| LogP | 3.57 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.535 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T+ |
| Risk Phrases | R26/27/28 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | EK7907100 |
| Precursor 5 | |
|---|---|
| DownStream 4 | |
|
Endothelial protective genes induced by statin are mimicked by ERK5 activation as triggered by a drug combination of FTI-277 and GGTI-298.
Biochim. Biophys. Acta 1850 , 1415-25, (2015) Statins are potent inhibitors of cholesterol biosynthesis and are clinically beneficial in preventing cardiovascular diseases, however, the therapeutic utility of these drugs is limited by myotoxicity... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: Chemometrics application.
J. Pharm. Biomed. Anal. 100 , 243-53, (2014) Red yeast rice (RYR) is prepared by fermenting rice with various strains of the yeast Monascus spp of the Aspergillaceae family. Depending on the Monascus strains and the fermentation conditions, the ... |
| ML 236B |
| Mevastatinum [INN-Latin] |
| mevastatin |
| Compactin (penicillium) |
| Antibiotic ML 236B |
| L66 AU IUTJ EOVY2&1 H1 G2- FT6OVTJ DQ &&stereoisomer |
| Mevastatin (Compactin) |
| MFCD05662341 |
| Statin I |
| [(1S,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2S)-2-methylbutanoate |
| (+)-Compactin |
| ML 236 B |
| (1S,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-7-methyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl (2S)-2-methylbutanoate |
| Butanoic acid, 2-methyl-, (1S,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester, (2S)- |
| 2b-Methyl-8a-(2-methyl-1-oxobutoxy)mevinic acid lactone |
| ML-236B |
| (1S,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate |
| 7-[1,2,6,7,8,8a-Hexahydro-2-methyl-8-(methylbutyryloxy)naphthyl]-3-hydroxyheptan-5-olide |
| [1S-[1a(R*),7b,8b(2S*,4S*),8ab]]-2-Methylbutanoic acid 1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester |
| Compactin |
| Lovastatin Impurity 1 |
![(1S,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-((2R,4R)-tetrahydro-4-methoxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthyl (2S)-2-methylbutyrate Structure](https://image.chemsrc.com/caspic/220/84751-53-1.png) CAS#:84751-53-1
CAS#:84751-53-1![methyl 3,5-dihydroxy-7-[(1'S,2'S,8'S,8a'S)-2'methyl-8'-[(S)-2-methylbutanoyloxy]-1',2',3',7',8',8a'-hexahydro-1'-naphthyl]heptanoate Structure](https://image.chemsrc.com/caspic/474/79814-60-1.png) CAS#:79814-60-1
CAS#:79814-60-1 CAS#:84131-75-9
CAS#:84131-75-9 CAS#:84173-21-7
CAS#:84173-21-7 CAS#:2906-93-6
CAS#:2906-93-6 CAS#:81093-37-0
CAS#:81093-37-0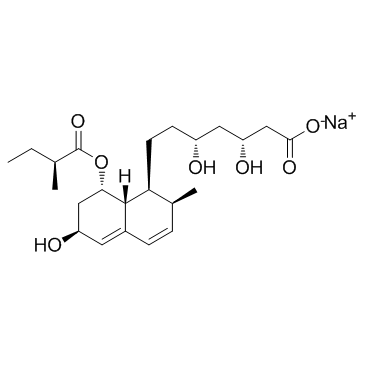 CAS#:81131-70-6
CAS#:81131-70-6 CAS#:67-64-1
CAS#:67-64-1 CAS#:75-65-0
CAS#:75-65-0
