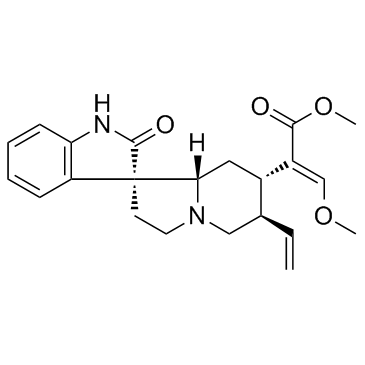
Rhynchophylline
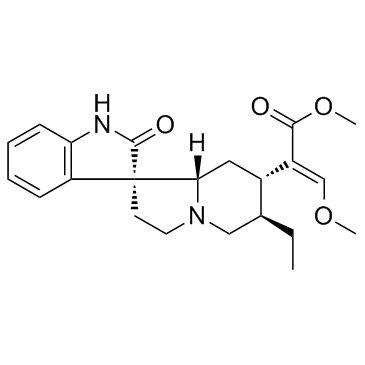
Rhynchophylline structure
|
Common Name | Rhynchophylline | ||
|---|---|---|---|---|
| CAS Number | 76-66-4 | Molecular Weight | 384.469 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 560.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H28N2O4 | Melting Point | 216°; mp 197-199° (Ban et al., loc. cit.) | |
| MSDS | N/A | Flash Point | 293.0±30.1 °C | |
Use of RhynchophyllineRhyncholphylline, an alkaloid isolated from Uncaria, shows potent inhibition of lipopolysaccharide (LPS)-induced NO production in rat primary microglial cells.IC50 value:Target:In vitro: Rhyncholphylline effectively suppresses release of proinflammatory cytokines in LPS-activated microglial cells and the underling molecular mechanism for the inhibition of microglial activation; Attenuated LPS-induced production of proinflammatory cytokines such as TNF-α and IL-1β as well as NO in mouse N9 microglial cells [1]. Rhynchophylline exerts it protective action against ischemia-induced neuronal damage by preventing NMDA, muscarinic M1, and 5-HT2 receptors-mediated neurotoxicity during ischemia [3].In vivo: The neuroprotective effect of rhynchophylline was investigated in a stroke model. Following pMCAO, rhynchophylline treatment not only ameliorated neurological deficits, infarct volume and brain edema, but also increased claudin-5 and BDNF expressions (p < 0.05). Moreover, rhynchophylline could activate PI3K/Akt/mTOR signaling while inhibiting TLRs/NF-κB pathway [2]. |
| Name | Rhynchophylline |
|---|---|
| Synonym | More Synonyms |
| Description | Rhyncholphylline, an alkaloid isolated from Uncaria, shows potent inhibition of lipopolysaccharide (LPS)-induced NO production in rat primary microglial cells.IC50 value:Target:In vitro: Rhyncholphylline effectively suppresses release of proinflammatory cytokines in LPS-activated microglial cells and the underling molecular mechanism for the inhibition of microglial activation; Attenuated LPS-induced production of proinflammatory cytokines such as TNF-α and IL-1β as well as NO in mouse N9 microglial cells [1]. Rhynchophylline exerts it protective action against ischemia-induced neuronal damage by preventing NMDA, muscarinic M1, and 5-HT2 receptors-mediated neurotoxicity during ischemia [3].In vivo: The neuroprotective effect of rhynchophylline was investigated in a stroke model. Following pMCAO, rhynchophylline treatment not only ameliorated neurological deficits, infarct volume and brain edema, but also increased claudin-5 and BDNF expressions (p < 0.05). Moreover, rhynchophylline could activate PI3K/Akt/mTOR signaling while inhibiting TLRs/NF-κB pathway [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 560.8±50.0 °C at 760 mmHg |
| Melting Point | 216°; mp 197-199° (Ban et al., loc. cit.) |
| Molecular Formula | C22H28N2O4 |
| Molecular Weight | 384.469 |
| Flash Point | 293.0±30.1 °C |
| Exact Mass | 384.204895 |
| PSA | 67.87000 |
| LogP | 3.31 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.596 |
| InChIKey | DAXYUDFNWXHGBE-UHFFFAOYSA-N |
| SMILES | CCC1CN2CCC3(C(=O)Nc4ccccc43)C2CC1C(=COC)C(=O)OC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S24/25 |
|---|---|
| RTECS | GN1600000 |
| HS Code | 29399990 |
|
~97% 
Rhynchophylline CAS#:76-66-4 |
| Literature: Wanner, Martin J.; Ingemann, Steen; Van Maarseveen, Jan H.; Hiemstra, Henk European Journal of Organic Chemistry, 2013 , # 6 p. 1100 - 1106 |
|
~% 
Rhynchophylline CAS#:76-66-4 |
| Literature: European Journal of Organic Chemistry, , # 6 p. 1100 - 1106 |
|
~% 
Rhynchophylline CAS#:76-66-4 |
| Literature: European Journal of Organic Chemistry, , # 6 p. 1100 - 1106 |
|
~% 
Rhynchophylline CAS#:76-66-4 |
| Literature: European Journal of Organic Chemistry, , # 6 p. 1100 - 1106 |
|
~% 
Rhynchophylline CAS#:76-66-4 |
| Literature: European Journal of Organic Chemistry, , # 6 p. 1100 - 1106 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| Methyl-(7β,16E,20α)-16-(methoxymethyliden)-2-oxocorynoxan-17-oat |
| Methyl (7β,16E,20α)-16-(methoxymethylene)-2-oxocorynoxan-17-oate |
| Methyl (16E)-16-(methoxymethylene)-2-oxocorynoxan-17-oate |
| Mitrinermine |
| Rhyncophylline |
| mitrinermin |
| Methyl (2E)-2-[(3R,6'R,7'S,8a'S)-6'-ethyl-2-oxo-1,2,2',3',6',7',8',8a'-octahydro-5'H-spiro[indole-3,1'-indolizin]-7'-yl]-3-methoxyacrylate |
| Methyl (7β,16E,20α)-2-hydroxy-17-methoxy-1,2-didehydrocorynox-16-en-16-carboxylate |
| Spiro[3H-indole-3,1'(5'H)-indolizine]-7'-acetic acid, 6'-ethyl-1,2,2',3',6',7',8',8'a-octahydro-α-(methoxymethylene)-2-oxo-, methyl ester, (αE,3S,6'S,7'S,8'aS)- |
| methyl (7β,16E,20α)-16-(methoxymethylidene)-2-oxocorynoxan-17-oate |
| Spiro[3H-indole-3,1'(5'H)-indolizine]-7'-acetic acid, 6'-ethyl-1,2,2',3',6',7',8',8'a-octahydro-α-(methoxymethylene)-2-oxo-, methyl ester, (αE,3R,6'R,7'S,8'aS)- |
| Spiro[3H-indole-3,1'(5'H)-indolizine]-7'-acetic acid, 6'-ethyl-2',3',6',7',8',8'a-hexahydro-2-hydroxy-α-(methoxymethylene)-, methyl ester, (αE,3R,6'R,7'S,8'aS)- |
| rhynchophyllin |
| RHYNCHOLPHYLLINE |