4-Phenylpiperidine
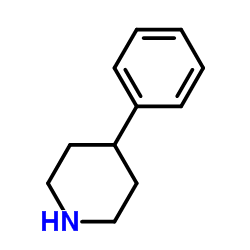
4-Phenylpiperidine structure
|
Common Name | 4-Phenylpiperidine | ||
|---|---|---|---|---|
| CAS Number | 771-99-3 | Molecular Weight | 161.243 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 259.9±29.0 °C at 760 mmHg | |
| Molecular Formula | C11H15N | Melting Point | 61-65 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 113.0±19.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 4-Phenylpiperidine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 259.9±29.0 °C at 760 mmHg |
| Melting Point | 61-65 °C(lit.) |
| Molecular Formula | C11H15N |
| Molecular Weight | 161.243 |
| Flash Point | 113.0±19.7 °C |
| Exact Mass | 161.120453 |
| PSA | 12.03000 |
| LogP | 2.39 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.522 |
| InChIKey | UTBULQCHEUWJNV-UHFFFAOYSA-N |
| SMILES | c1ccc(C2CCNCC2)cc1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39-S45-S37/39-S36/37 |
| RIDADR | UN 2922 8/PG 2 |
| WGK Germany | 3 |
| RTECS | TM2625000 |
| HS Code | 2933399090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
A strategy for isotope containment during radiosynthesis--devolatilisation of bromobenzene by fluorous-tagging-Ir-catalysed borylation en route to the 4-phenylpiperidine pharmacophore.
Org. Biomol. Chem. 6 , 4093-4095, (2008) Syntheses of two 4-phenylpiperidines from bromobenzene have been developed involving anchoring to a fluorous-tag, Ir-catalysed borylation, Pd- and Co-catalysed elaboration then traceless cleavage. Alt... |
|
|
Structure-activity studies of morphine fragments. I. 4-alkyl-4-(m-hydroxy-phenyl)-piperidines.
Mol. Pharmacol. 34(3) , 363-76, (1988) The 4-(m-OH-phenyl)piperidines are a flexible fragment of the morphine/benzomorphan fused-ring opioids. Analogs in this family were synthesized with varying 4-alkyl substituents increasing in bulk fro... |
|
|
Radical-mediated nitrile translocation as the key step in the stereoselective transformation of 2-(4-chloro-2-cyanobutyl)aziridines to methyl cis-(1-arylmethyl-4-phenylpiperidin-2-yl)acetates.
Org. Biomol. Chem. 10(16) , 3308-14, (2012) Non-activated 2-(4-chloro-2-cyano-2-phenylbutyl)aziridines were used as building blocks for the stereoselective synthesis of novel cis-2-cyanomethyl-4-phenylpiperidines via a microwave-assisted azirid... |
| Piperidine,4-phenyl |
| 4-phenylpiperazine |
| MFCD00005957 |
| 4-Ph-piperdine |
| EINECS 212-243-1 |
| 4-phenyl-piperidine |
 CAS#:38025-45-5
CAS#:38025-45-5 CAS#:123387-49-5
CAS#:123387-49-5 CAS#:43064-12-6
CAS#:43064-12-6 CAS#:84473-59-6
CAS#:84473-59-6 CAS#:939-23-1
CAS#:939-23-1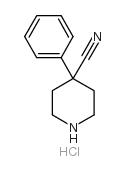 CAS#:51304-58-6
CAS#:51304-58-6 CAS#:10338-69-9
CAS#:10338-69-9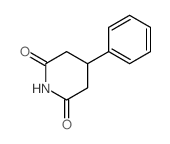 CAS#:14149-31-6
CAS#:14149-31-6 CAS#:62143-51-5
CAS#:62143-51-5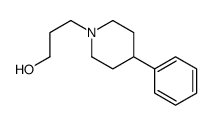 CAS#:15037-36-2
CAS#:15037-36-2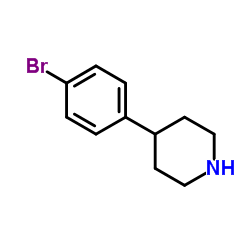 CAS#:80980-89-8
CAS#:80980-89-8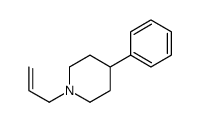 CAS#:31414-54-7
CAS#:31414-54-7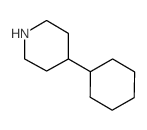 CAS#:14446-73-2
CAS#:14446-73-2 CAS#:136534-70-8
CAS#:136534-70-8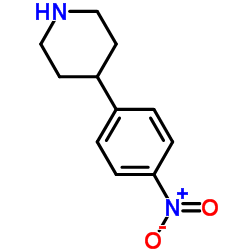 CAS#:26905-03-3
CAS#:26905-03-3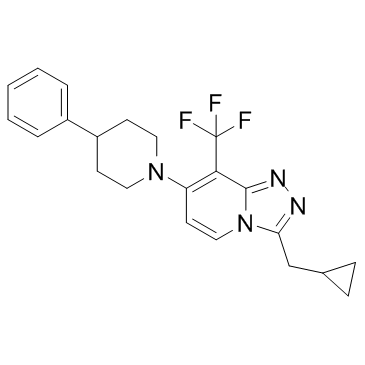 CAS#:1254977-87-1
CAS#:1254977-87-1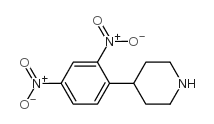 CAS#:120447-45-2
CAS#:120447-45-2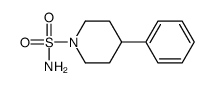 CAS#:16168-22-2
CAS#:16168-22-2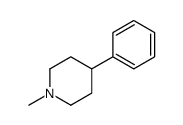 CAS#:774-52-7
CAS#:774-52-7
