Hydroxysafflor yellow A
Modify Date: 2025-08-22 19:29:28
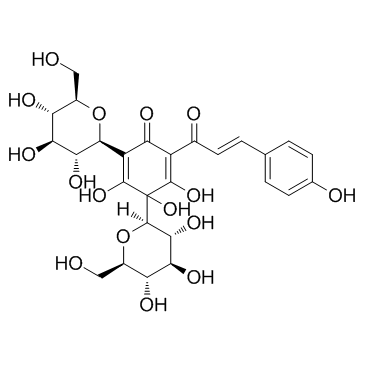
Hydroxysafflor yellow A structure
|
Common Name | Hydroxysafflor yellow A | ||
|---|---|---|---|---|
| CAS Number | 78281-02-4 | Molecular Weight | 612.533 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 1015.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C27H32O16 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 334.0±27.8 °C | |
Use of Hydroxysafflor yellow AHydroxysafflor yellow A is a flavonoid derived and isolated from traditional Chinese medicine Carthamus tinctorius L., possesses anti-tumor activity. IC50 value:Target: in vitro: HYSA could inhibit LPS-induced VSMCs proliferation and migration, accompanied by the downregulated levels of several key pro-inflammatory cytokines, including TNF-α, IL-6, and IL-8. We further showed that HYSA inhibited LPS-induced upregulation of TLR-4 expression as well as the activation of Rac1/Akt pathway [1]. HSYA protected EC viability against LPS-induced injury (P<0.05). LPS-induced NF-κB p65 subunit DNA binding (P<0.01) and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor -α (I-κB-α) phosphorylation was inhibited by HSYA. HSYA attenuated LPS triggered ICAM-1 and E-selectin mRNA levels elevation and phosphorylation of p38 MAPK or c-Jun N-terminal kinase MAPK [2]. HSYA inhibited the proliferation of 3T3-L1 preadipocytes and cell viability greatly decreased in a dose and time dependent manner. HSYA (1 mg/l) notably reduced the amount of intracellular lipid and triglyceride content in adipocytes by 21.3 % (2.13 ± 0.36 vs 2.71 ± 0.40, P < 0.01) and 22.6 % (1.33 ± 0.07 vs 1.72 ± 0.07, P < 0.01) on days 8 following the differentiation, respectively [3]. in vivo: HSYA treatment ameliorated serum biochemical indicators by reducing the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronan (HA), laminin (LN), and type III precollagen (III-C) in rats [4]. |
| Name | Hydroxysafflor yellow A |
|---|---|
| Synonym | More Synonyms |
| Description | Hydroxysafflor yellow A is a flavonoid derived and isolated from traditional Chinese medicine Carthamus tinctorius L., possesses anti-tumor activity. IC50 value:Target: in vitro: HYSA could inhibit LPS-induced VSMCs proliferation and migration, accompanied by the downregulated levels of several key pro-inflammatory cytokines, including TNF-α, IL-6, and IL-8. We further showed that HYSA inhibited LPS-induced upregulation of TLR-4 expression as well as the activation of Rac1/Akt pathway [1]. HSYA protected EC viability against LPS-induced injury (P<0.05). LPS-induced NF-κB p65 subunit DNA binding (P<0.01) and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor -α (I-κB-α) phosphorylation was inhibited by HSYA. HSYA attenuated LPS triggered ICAM-1 and E-selectin mRNA levels elevation and phosphorylation of p38 MAPK or c-Jun N-terminal kinase MAPK [2]. HSYA inhibited the proliferation of 3T3-L1 preadipocytes and cell viability greatly decreased in a dose and time dependent manner. HSYA (1 mg/l) notably reduced the amount of intracellular lipid and triglyceride content in adipocytes by 21.3 % (2.13 ± 0.36 vs 2.71 ± 0.40, P < 0.01) and 22.6 % (1.33 ± 0.07 vs 1.72 ± 0.07, P < 0.01) on days 8 following the differentiation, respectively [3]. in vivo: HSYA treatment ameliorated serum biochemical indicators by reducing the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronan (HA), laminin (LN), and type III precollagen (III-C) in rats [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 1015.8±65.0 °C at 760 mmHg |
| Molecular Formula | C27H32O16 |
| Molecular Weight | 612.533 |
| Flash Point | 334.0±27.8 °C |
| Exact Mass | 612.169006 |
| PSA | 295.36000 |
| LogP | 2.98 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.797 |
| InChIKey | IAVUBSCVWHLRGE-MFMRYLTCSA-N |
| SMILES | O=C1C(=C(O)C=Cc2ccc(O)cc2)C(O)=C(C2OC(CO)C(O)C(O)C2O)C(=O)C1(O)C1OC(CO)C(O)C(O)C1O |
| Safety Phrases | 24/25 |
|---|
| hydroxysaffloryellowA |
| 3,4,5-Trihydroxy-2-[(2E)-3-(4-hydroxyphenyl)-2-propenoyl]-4-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-2,5-cyclohexadien-1-one |
| safflomin-A |

