Mensacarcin
Modify Date: 2025-09-27 11:22:01
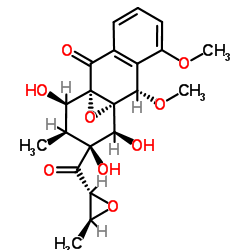
Mensacarcin structure
|
Common Name | Mensacarcin | ||
|---|---|---|---|---|
| CAS Number | 808750-39-2 | Molecular Weight | 420.410 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 656.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C21H24O9 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 232.9±25.0 °C | |
Use of MensacarcinMensacarcin, a highly complex polyketide, strongly inhibits cell growth universally in cancer cell lines and potently induces apoptosis in melanoma cells. Mensacarcin targets to mitochondria, affects energy metabolism in mitochondria, and activates caspase-dependent apoptotic pathways. Mensacarcin, an antibiotic, can be used as a cytotoxic component of antibody-drug conjugates (ADCs)[1][2]. |
| Name | (1S,9S,10S,11S,12S,13S,14R)-11,12,14-Trihydroxy-7,9-dimethoxy-13-methyl-12-{[(2R,3S)-3-methyl-2-oxiranyl]carbonyl}-15-oxatetracyclo[8.4.1.01,10.03,8]pentadeca-3,5,7-trien-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | Mensacarcin, a highly complex polyketide, strongly inhibits cell growth universally in cancer cell lines and potently induces apoptosis in melanoma cells. Mensacarcin targets to mitochondria, affects energy metabolism in mitochondria, and activates caspase-dependent apoptotic pathways. Mensacarcin, an antibiotic, can be used as a cytotoxic component of antibody-drug conjugates (ADCs)[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Traditional Cytotoxic Agents |
| In Vitro | Mensacarcin (0-100 μM; 24 hours) exhibits general cytostatic but type-specific cytotoxic effects for melanoma cells[1]. Mensacarcin (2-50 μM; 15 hours) induces rapid apoptotic cell death in melanoma cells[1]. Mensacarcin exhibits potent cytostatic properties (mean of 50% growth inhibition=0.2 μM) in almost all cell lines of the National Cancer Institute (NCI)-60 cell line screen and relatively selective cytotoxicity against melanoma cells. Mensacarcin is a highly oxygenated polyketide that was first isolated from soil-dwelling Streptomyces bacteria. Mensacarcin impairs mitochondrial function in melanoma cells[1]. Cell Viability Assay[1] Cell Line: SK-Mel-28 and SK-Mel-5 melanoma cells, HCT-116 colon cancer cells Concentration: 0.01, 0.1, 1, 10, 100 μM Incubation Time: 24 hours Result: Induced concentration- and time-dependent cell death in the two tested melanoma cell lines. HCT-116 colon carcinoma cells were strongly inhibited. Western Blot Analysis[1] Cell Line: SK-Mel-28, SK-Mel-5 cells Concentration: 2, 10, 50 μM Incubation Time: 15 hours Result: Induced the formation of 89-kDa PARP-1 fragments as well as caspase-3 activation in SK-Mel-28 and SK-Mel-5 beginning between 6 and 15 h after exposure. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 656.8±55.0 °C at 760 mmHg |
| Molecular Formula | C21H24O9 |
| Molecular Weight | 420.410 |
| Flash Point | 232.9±25.0 °C |
| Exact Mass | 420.142029 |
| LogP | 5.48 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.647 |
| InChIKey | WWNXYRCJJRRWAQ-ALCXHWRFSA-N |
| SMILES | COc1cccc2c1C(OC)C13OC1(C2=O)C(O)C(C)C(O)(C(=O)C1OC1C)C3O |
| (1S,9S,10S,11S,12S,13S,14R)-11,12,14-Trihydroxy-7,9-dimethoxy-13-methyl-12-{[(2R,3S)-3-methyl-2-oxiranyl]carbonyl}-15-oxatetracyclo[8.4.1.01,10.03,8]pentadeca-3,5,7-trien-2-one |
| 4a,9a-Epoxyanthracen-9(10H)-one, 1,2,3,4-tetrahydro-1,3,4-trihydroxy-5,10-dimethoxy-2-methyl-3-[[(2R,3S)-3-methyloxiranyl]carbonyl]-, (1R,2S,3S,4S,4aS,9aS,10S)- |