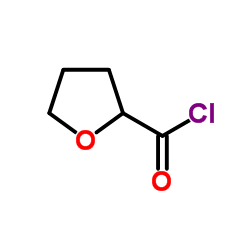
Alfuzosin
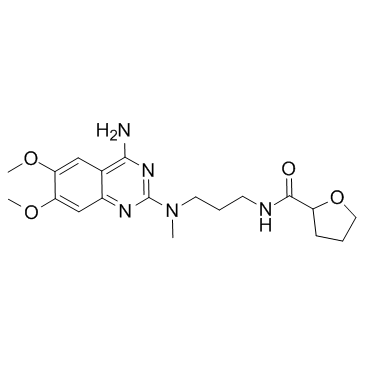
Alfuzosin structure
|
Common Name | Alfuzosin | ||
|---|---|---|---|---|
| CAS Number | 81403-80-7 | Molecular Weight | 389.449 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C19H27N5O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of AlfuzosinAlfuzosin is an α1 adrenergic receptor antagonist used to treat benign prostatic hyperplasia (BPH).Target: α1 adrenergic receptorAlfuzosin, a new quinazoline derivative, acts as a selective and competitive antagonist of alpha 1-adrenoceptor-mediated contraction of prostatic, prostatic capsule, bladder base and proximal urethral smooth muscle, thereby reducing the tone of these structures. Consequently, urethral pressure and resistance, bladder outlet resistance, bladder instability and symptoms associated with benign prostatic hyperplasia are reduced. A limited range of clinical studies have shown oral alfuzosin to be more effective than placebo (in studies of < or = 6 months duration), to have sustained effects on long term administration (< or = 30 months), and to be comparable with the alpha 1-adrenoceptor antagonist prazosin, in the symptomatic treatment of benign prostatic hyperplasia.Oral alfuzosin 7.5 to 10 mg/day in divided doses appears to be a promising first-line agent for symptomatic treatment of noncomplicated mild to moderate benign prostatic hyperplasia in patients with a high dynamic component to their obstruction. In addition, alfuzosin offers an alternative to prostatectomy (the current 'gold standard') in patients who require surgery but are unfit for this treatment, and in patients requiring symptomatic relief while awaiting surgery. |
| Name | alfuzosin |
|---|---|
| Synonym | More Synonyms |
| Description | Alfuzosin is an α1 adrenergic receptor antagonist used to treat benign prostatic hyperplasia (BPH).Target: α1 adrenergic receptorAlfuzosin, a new quinazoline derivative, acts as a selective and competitive antagonist of alpha 1-adrenoceptor-mediated contraction of prostatic, prostatic capsule, bladder base and proximal urethral smooth muscle, thereby reducing the tone of these structures. Consequently, urethral pressure and resistance, bladder outlet resistance, bladder instability and symptoms associated with benign prostatic hyperplasia are reduced. A limited range of clinical studies have shown oral alfuzosin to be more effective than placebo (in studies of < or = 6 months duration), to have sustained effects on long term administration (< or = 30 months), and to be comparable with the alpha 1-adrenoceptor antagonist prazosin, in the symptomatic treatment of benign prostatic hyperplasia.Oral alfuzosin 7.5 to 10 mg/day in divided doses appears to be a promising first-line agent for symptomatic treatment of noncomplicated mild to moderate benign prostatic hyperplasia in patients with a high dynamic component to their obstruction. In addition, alfuzosin offers an alternative to prostatectomy (the current 'gold standard') in patients who require surgery but are unfit for this treatment, and in patients requiring symptomatic relief while awaiting surgery. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C19H27N5O4 |
| Molecular Weight | 389.449 |
| Exact Mass | 389.206299 |
| PSA | 111.83000 |
| LogP | -1.00 |
| Index of Refraction | 1.621 |
| InChIKey | WNMJYKCGWZFFKR-UHFFFAOYSA-N |
| SMILES | COc1cc2nc(N(C)CCCNC(=O)C3CCCO3)nc(N)c2cc1OC |
| Storage condition | 2-8℃ |
|
~80% 
Alfuzosin CAS#:81403-80-7 |
| Literature: UNICHEM LABORATORIES LIMITED Patent: WO2008/84493 A2, 2008 ; Location in patent: Page/Page column 9 ; |
|
~71% 
Alfuzosin CAS#:81403-80-7 |
| Literature: AUROBINDO PHARMA LIMITED Patent: WO2007/74364 A1, 2007 ; Location in patent: Page/Page column 9 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2010/10058 A1, ; Page/Page column 14 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2006/90268 A2, ; Page/Page column 28; 30-31 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2006/30449 A1, ; Page/Page column 5-6 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2007/74364 A1, ; Page/Page column 8 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2008/15525 A2, ; Page/Page column 7; 10-11 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2009/7987 A1, ; Page/Page column 11 ; |
|
~% 
Alfuzosin CAS#:81403-80-7 |
| Literature: WO2006/90268 A2, ; Page/Page column 30 ; |
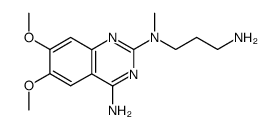
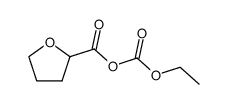
| Precursor 9 | |
|---|---|
| DownStream 1 | |
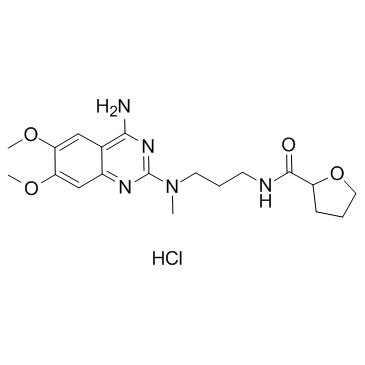
| Alfuzosin |
| Xatral |
| Urion |
| ALFUZOCIN |
| SL 77499-10 |
| ALFUZOSIN HYDROCLORIDE |
| 2-furancarboxamide, N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro- |
| MFCD00865792 |
| ALFUZOSINE |
| Alfuzosion |
| Alfoten |
| N-{3-[(4-amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino]propyl}tetrahydrofuran-2-carboxamide |
| N-{3-[(4-Amino-6,7-dimethoxy-2-quinazolinyl)(methyl)amino]propyl}tetrahydro-2-furancarboxamide |
| Afluzosin |
![N-[3-(methylamino)propyl]oxolane-2-carboxamide structure](https://image.chemsrc.com/caspic/431/81403-67-0.png)


![(R,S)-N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide acetate structure](https://image.chemsrc.com/caspic/392/1100050-87-0.png)