Iodomethane-d3
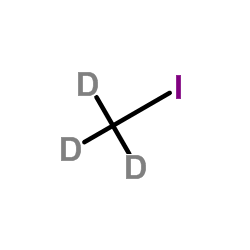
Iodomethane-d3 structure
|
Common Name | Iodomethane-d3 | ||
|---|---|---|---|---|
| CAS Number | 865-50-9 | Molecular Weight | 144.958 | |
| Density | 2.2±0.1 g/cm3 | Boiling Point | 40.3±3.0 °C at 760 mmHg | |
| Molecular Formula | CD3I | Melting Point | -66.5ºC(lit.) | |
| MSDS | USA | Flash Point | 7.8±10.7 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
| Name | trideuterio(iodo)methane |
|---|---|
| Synonym | More Synonyms |
| Density | 2.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 40.3±3.0 °C at 760 mmHg |
| Melting Point | -66.5ºC(lit.) |
| Molecular Formula | CD3I |
| Molecular Weight | 144.958 |
| Flash Point | 7.8±10.7 °C |
| Exact Mass | 144.946762 |
| LogP | 1.50 |
| Vapour density | 4.89 (vs air) |
| Vapour Pressure | 437.1±0.1 mmHg at 25°C |
| Index of Refraction | 1.528 |
| InChIKey | INQOMBQAUSQDDS-FIBGUPNXSA-N |
| SMILES | CI |
| Storage condition | 0-6°C |
| Stability | Stable. Incompatible with strong bases, strong oxidizing agents. Moisture and light-sensitive. |
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H331-H312-H315-H317-H334-H335-H351-H371 |
| Precautionary Statements | P260-P280-P301 + P310-P308 + P311-P311 |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R21;R23/25;R37/38;R40 |
| Safety Phrases | S36/37-S38-S45 |
| RIDADR | UN 2644 6.1/PG 1 |
| WGK Germany | 3 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| HS Code | 28459010 |
|
~68% 
Iodomethane-d3 CAS#:865-50-9 |
| Literature: Shiner, V. J.; Ensinger, Mark W.; Rutkowske, Randy D. Journal of the American Chemical Society, 1987 , vol. 109, # 3 p. 804 - 809 |
|
~% 
Iodomethane-d3 CAS#:865-50-9 |
| Literature: Zhurnal Obshchei Khimii, , vol. 24, p. 1321,1322; engl.Ausg.S.1303,1304 |
|
~% 
Iodomethane-d3 CAS#:865-50-9 |
| Literature: Canadian Journal of Chemistry, , vol. 32, p. 1 |
|
~% 
Iodomethane-d3 CAS#:865-50-9 |
| Literature: Magnetic Resonance in Chemistry, , vol. 28, # 5 p. 389 - 396 |
|
~% 
Iodomethane-d3 CAS#:865-50-9 |
| Literature: Russian Journal of General Chemistry, , vol. 64, # 6.1 p. 860 - 868 Zhurnal Obshchei Khimii, , vol. 64, # 6 p. 956 - 965 |
| Precursor 5 | |
|---|---|
| DownStream 9 | |
|
Mitigating 1,3-dichloropropene, chloropicrin, and methyl iodide emissions from fumigated soil with reactive film.
Environ. Sci. Technol. 46(11) , 6143-9, (2012) Implicated as a stratospheric ozone-depleting compound, methyl bromide (MeBr) is being phased out despite being considered to be the most effective soil fumigant. Its alternatives, i.e., 1,3-dichlorop... |
|
|
Effects of depletion of glutathione on abscisic acid- and methyl jasmonate-induced stomatal closure in Arabidopsis thaliana.
Biosci. Biotechnol. Biochem. 76(11) , 2032-7, (2012) Glutathione (GSH) is involved in abscisic acid (ABA)- and methyl jasmonate (MeJA)-induced stomatal closure in Arabidopsis thaliana. In this study, we examined the effects of GSH-decreasing chemicals, ... |
|
|
A-band methyl halide dissociation via electronic curve crossing as studied by electron energy loss spectroscopy.
J. Chem. Phys. 133(5) , 054304, (2010) Excitation of the A-band low-lying electronic states in the methyl halides, CH(3)I, CH(3)Br, CH(3)Cl, and CH(3)F, has been investigated for the (n-->sigma*) transitions, using electron energy loss spe... |
| EINECS 212-744-5 |
| trideuterioiodomethane |
| Iodomethane-12C,d3 |
| IODOMETHANE D3 |
| [d3]-iodomethane |
| Iodo(H)methane |
| iodo(2H3)methane |
| Methyl iodide (D3) |
| Methyl-d3 Iodide |
| IODOMETHANE-12C-D3 |
| MFCD00001074 |
| Iodomethane-d3 |
| deuteroiodomethane |
| [2H3]methyl iodide |
| deuterated iodomethane |
| Methane-d, iodo- |
| Methyl-12C,d3 iodide |
| METHYL IODIDE-D3 |
| Trideuteromethyl iodide |




![N-[2-(S)-Methyl-d3-butyryl]-4-(S)-phenylmethyl-2-oxazolidinone structure](https://image.chemsrc.com/caspic/151/1073232-99-1.png) CAS#:1073232-99-1
CAS#:1073232-99-1 CAS#:106776-17-4
CAS#:106776-17-4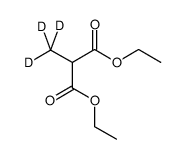 CAS#:54840-57-2
CAS#:54840-57-2![Methyl 4-hydroxy-2-methyl-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide-d3 structure](https://image.chemsrc.com/caspic/111/942047-62-3.png) CAS#:942047-62-3
CAS#:942047-62-3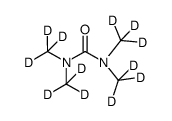 CAS#:51219-89-7
CAS#:51219-89-7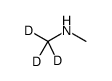 CAS#:20786-94-1
CAS#:20786-94-1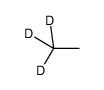 CAS#:2031-95-0
CAS#:2031-95-0 CAS#:26351-03-1
CAS#:26351-03-1 CAS#:26351-04-2
CAS#:26351-04-2
