PT-262
Modify Date: 2025-09-12 16:53:11
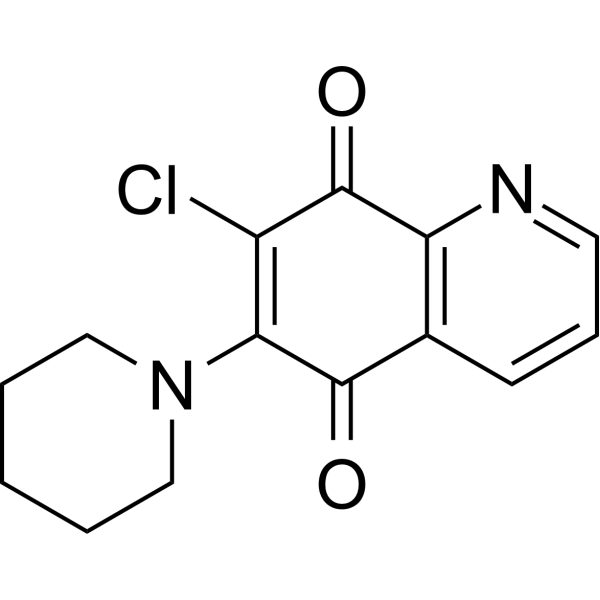
PT-262 structure
|
Common Name | PT-262 | ||
|---|---|---|---|---|
| CAS Number | 86811-36-1 | Molecular Weight | 276.72 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H13ClN2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of PT-262PT-262 is a potent ROCK inhibitor with an IC50 value of around 5 μM. PT-262 induces the loss of mitochondrial membrane potential and elevates the caspase-3 activation and apoptosis. PT-262 inhibits the ERK and CDC2 phosphorylation via a p53-independent pathway. PT-262 blocks cytoskeleton function and cell migration. PT-262 has anti-cancer activity[1][2]. |
| Name | PT-262 |
|---|
| Description | PT-262 is a potent ROCK inhibitor with an IC50 value of around 5 μM. PT-262 induces the loss of mitochondrial membrane potential and elevates the caspase-3 activation and apoptosis. PT-262 inhibits the ERK and CDC2 phosphorylation via a p53-independent pathway. PT-262 blocks cytoskeleton function and cell migration. PT-262 has anti-cancer activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
ROCK:5 μM (IC50) ERK CDK2 |
| In Vitro | PT-262 (5-40 μM; 24 h) induces cytotoxicity and proliferation inhibition in human lung cancer cells[1]. PT-262 (2-20 μM; 4-24 h) induces caspase-3 activation, mitochondrial dysfunction and apoptosis in lung cancer cells[1]. PT-262 (10-20 μM; 24 h) induces the accumulation of G2/M phases in both the p53-wild type and p53-null lung cancer cells, and inhibits the phosphorylation of CDC2 proteins[1]. PT-262 (0-10 μM; 24 h) represses ERK phosphorylation in lung cancer cells[1]. PT-262 (2 μM; 24 h) induces the cytoskeleton alteration and cell elongation in lung carcinoma A549 cells[2]. PT-262 (2-10 μM; 6 h) significantly blocks the cell migration in a concentration-dependent manner[2]. Cell Viability Assay[1] Cell Line: A549 cells Concentration: 5-40 μM Incubation Time: 24 h Result: Reduced the cell viability via a concentration-dependent manner in A549 cells. The IC50 value toward human normal lung fibroblast was >20 μM. Apoptosis Analysis[1] Cell Line: A549 cells Concentration: 2-20 μM Incubation Time: 4-24 h Result: The apoptotic cells were increased after treatment at 10 μM for 8-24 h. The active forms of caspase-3 (12 and 17 kD) were induced following treatment with 2-20 μM for 24 h. Cell Cycle Analysis[1] Cell Line: A549 and H1299 cells Concentration: 10-20 μM Incubation Time: 24 h Result: Significantly decreased the G1 fractions while increased the G2/M fractions in both A549 and H1299 cells with 10 μM for 24 h. Decreased the protein levels of cyclin B1 and phospho-CDC2 at Thr14, Tyr15, and Thr161 via a concentration-dependent manner in A549 cells. Western Blot Analysis[1] Cell Line: A549 cells Concentration: 0-10 μM Incubation Time: 24 h Result: Significantly inhibited the phosphorylation of ERK. |
| References |
| Molecular Formula | C14H13ClN2O2 |
|---|---|
| Molecular Weight | 276.72 |
| InChIKey | XBHXHCMFAUSIKK-UHFFFAOYSA-N |
| SMILES | O=C1C(N2CCCCC2)=C(Cl)C(=O)c2ncccc21 |