MK-8776(SCH 900776)
Modify Date: 2024-01-02 00:05:01

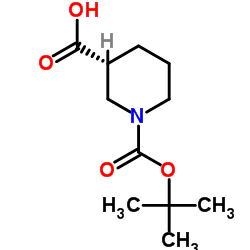
MK-8776(SCH 900776) structure
|
Common Name | MK-8776(SCH 900776) | ||
|---|---|---|---|---|
| CAS Number | 891494-63-6 | Molecular Weight | 376.254 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H18BrN7 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of MK-8776(SCH 900776)SCH900776 is a potent, selective and oral inhibitor of checkpoint kinase1 (Chk1) with an IC50 of 3 nM. It shows 50- and 500-fold selectivity over CDK2 andChk2, respectively. |
| Name | sch 900776 |
|---|---|
| Synonym | More Synonyms |
| Description | SCH900776 is a potent, selective and oral inhibitor of checkpoint kinase1 (Chk1) with an IC50 of 3 nM. It shows 50- and 500-fold selectivity over CDK2 andChk2, respectively. |
|---|---|
| Related Catalog | |
| Target |
Chk1:3 nM (IC50) Chk2:1500 nM (IC50) CDK2:160 nM (IC50) |
| In Vitro | SCH900776 (300 nM) shows potent inhibitory activities against phosphorylation at ser296-Chk1. SCH900776 (1 μM) causes a 30-fold decrease in the IC50 for hydroxyurea in MDA-MB-231 cells[1]. The Kd value of SCH 900776 for the CHK1 kinase domain is 2 nM. SCH 900776 exhibits an approximate EC50 of 60 nM in cells exposure to hydroxyurea. SCH 900776 induces dose-dependent suppression of CHK1 pS296 and concomitant accumulation of phospho-RPA signal in U2OS cells[2]. |
| In Vivo | SCH 900776 induces the γ-H2AX biomarker at 4 mg/kg (i.p.), and enhances tumor pharmacodynamic and regression responses in A2780 xenograft model. SCH 900776 (16 and 32 mg/kg, i.p.) induces incremental improvements in tumor response. Escalation of SCH 900776 dose to 20 and 50 mg/kg in combination with gemcitabine results in improvements in TTP 10× in the A2780 xenograft systems[2]. |
| Kinase Assay | The Kinase Profiler service is used to generate general selectivity data for SCH 900776 against a broad range of serine/threonine and tyrosine kinases. Assays are typically run at two concentrations of SCH 900776 (0.5 and 5 μM), at a fixed (10 μM) concentration of ATP. |
| Cell Assay | For cell growth assays, cells are seeded at low density (500-1000 cells) in 96-well plates and then incubated with drug for 24 h (8 wells per concentration). Following treatment, cells are washed and grown in fresh media for 5-7 days at 37°C. Prior to attaining confluence, cells are washed, lysed, and stained with Hoechst 33258. Fluorescence is read on a microplate spectrofluorometer. Results are expressed as mean and standard error for the concentration of drug that inhibited growth by 50%. |
| Animal Admin | For tumor implantation, specific cell lines are grown in vitro, washed once with PBS and resuspended in 50% Matrigel in PBS to a final concentration of 4×107 to 5×107 cells per mL. Nude mice are injected with 0.1 mL of this suspension subcutaneously in the flank region. Tumor length (L), width (W), and height (H) are measured by a caliper twice a week on each mouse and then used to calculate tumor volume using the formula: (L×W×H)/2. Animals (N=10) are randomized to treatment groups and treated intraperitoneally with either SCH 900776 (formulated in 20% hydroxypropyl β-cyclodextrin) or individual chemotherapeutic agents, formulated as recommended. Tumor volumes and body weights are measured during and after the treatment periods. Data are recorded as means±SEM before being normalized to starting volume. Time to progression to 10x starting volume (TTP 10x) is monitored in some experiments. For pharmacodynamic marker analyses in mice, tumors and adjacent skin are collected at necropsy, fixed overnight in 10% formalin, and washed/stored in 70% ethanol. For skin punch biopsies, an area of approximately 4 square inches is shaved. Rats are anesthetized using inhaled isofluorane and dogs are locally anesthetized using subcutaneous administration of lidocaine. Samples are collected using a 4 mm biopsy punch. Skin punches are fixed in 10% formalin overnight before washing/storage in 70% ethanol. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Molecular Formula | C15H18BrN7 |
| Molecular Weight | 376.254 |
| Exact Mass | 375.080688 |
| PSA | 86.06000 |
| LogP | 0.76 |
| Index of Refraction | 1.819 |
| Storage condition | -20℃ |
|
~90% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: SCHERING CORPORATION; CHRISTENSEN, Melodie, D.; KIM, Jungchul Patent: WO2011/119457 A1, 2011 ; Location in patent: Page/Page column 3 ; |
|
~% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: WO2013/39854 A1, ; |
|
~% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: WO2013/39854 A1, ; |
|
~% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: WO2013/39854 A1, ; |
|
~% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: WO2013/39854 A1, ; |
|
~% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: WO2013/39854 A1, ; |
|
~% 
MK-8776(SCH 900776) CAS#:891494-63-6 |
| Literature: WO2013/39854 A1, ; |
| Pyrazolo[1,5-a]pyrimidin-7-amine, 6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-[(3R)-3-piperidinyl]- |
| SCH 900776 |
| MK-8776 |
| 6-Bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-[(3R)-3-piperidinyl]pyrazolo[1,5-a]pyrimidin-7-amine |
| 6-Bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(3R)-3-piperidinylpyrazolo[1,5-a]pyrimidin-7-amine |
| SCH900776 |
![(R)-tert-butyl 3-(7-amino-6-bromo-3-(1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidin-5-yl)piperidine-1-carboxylate structure](https://www.chemsrc.com/caspic/209/1335211-24-9.png)


![[4,4'-Bi-1H-pyrazol]-3-amine, 1'-methyl structure](https://www.chemsrc.com/caspic/308/930286-91-2.png)