Dimethyloxalylglycine
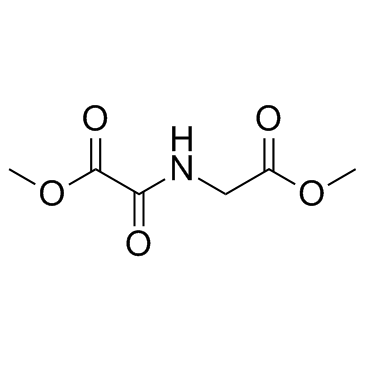
Dimethyloxalylglycine structure
|
Common Name | Dimethyloxalylglycine | ||
|---|---|---|---|---|
| CAS Number | 89464-63-1 | Molecular Weight | 175.139 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C6H9NO5 | Melting Point | 46-48ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of DimethyloxalylglycineDMOG (Dimethyloxallyl Glycine) is a cell-permeable and competitive inhibitor of HIF-1α prolyl hydroxylase (HIF-PH). |
| Name | Dimethyloxalylglycine |
|---|---|
| Synonym | More Synonyms |
| Description | DMOG (Dimethyloxallyl Glycine) is a cell-permeable and competitive inhibitor of HIF-1α prolyl hydroxylase (HIF-PH). |
|---|---|
| Related Catalog | |
| Target |
HIF-1α prolyl hydroxylase[1] |
| In Vitro | DMOG efficiently suppresses hydroxyproline synthesis in intact cells, but shows only weakly active in the microsomal system[1]. DMOG reduces FGF-2-induced proliferation and cyclin A expression by inhibiting prolyl hydroxylase activity in HPASMC[3]. |
| In Vivo | DMOG inhibits endogenous HIF inactivation, and induces angiogenesis in ischaemic skeletal muscles of mice[2]. Up-regulation of hypoxia-inducible factor-1α by DMOG enhances the cardioprotective effects of ischemic postconditioning in hyperlipidemic rats[4]. |
| Cell Assay | To analyze DNA synthesis as an index of cellular proliferation, VSMC are plated in 48-well plates (5,000 per square centimeter) in growth medium, incubated overnight, and serum-deprived (1% FCS) for 24 h. Replicate wells are then stored at −70°C for baseline (day 0) cell counts, and fresh medium with or without growth factors is added to the remaining wells, which are incubated 72-96 h in 20 or 5% O2. Days 0 and 3 or 4 cell counts are determined by lysing cells in a buffer containing a fluorescent dye, which has minimal fluorescence by itself but fluoresces when bound to DNA or RNA. Absolute cell numbers are calculated by comparing the fluorescence of specimens with that of a standard curve similarly prepared using a known number of cells. |
| Animal Admin | Two groups of mice (C57Bl6) are subjected to unilateral femoral artery ligation under fluothane inhalation anaesthesia (2% in O2). One group (n=11) receives dimethyloxalylglycine (DMOG) i.p. (8 mg in 0.5 mL saline) on days 1, 3, 5, 7 and 9 while the animals in the other group are injected with sterile saline (0.5 mL) at the same intervals (n=6). A third group is treated with DMOG without ligation (n=4) and four unoperated mice serve as controls. After 11 days mice are terminally anaesthetized and the extensor digitorum longus (EDL) and tibialis anterior (TA) muscles excised. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 46-48ºC |
| Molecular Formula | C6H9NO5 |
| Molecular Weight | 175.139 |
| Exact Mass | 175.048065 |
| PSA | 81.70000 |
| LogP | -0.56 |
| Appearance of Characters | Solid | white to off-white |
| Index of Refraction | 1.440 |
| Storage condition | Refrigerator |
| Water Solubility | H2O: >30mg/mL | Soluble in water at 30mg/ml. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2924199090 |
|
~88% 
Dimethyloxalylg... CAS#:89464-63-1 |
| Literature: Tetrahedron Letters, , vol. 50, # 9 p. 1045 - 1047 |
|
~99% 
Dimethyloxalylg... CAS#:89464-63-1 |
| Literature: EP1785418 A1, ; Page/Page column 66 ; |
|
~% 
Dimethyloxalylg... CAS#:89464-63-1 |
| Literature: Journal of Organic Chemistry, , vol. 66, # 19 p. 6369 - 6374 |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Anti-chondroitin sulfate proteoglycan 4-specific antibodies modify the effects of vemurafenib on melanoma cells differentially in normoxia and hypoxia.
Int. J. Oncol. 47 , 81-90, (2015) Chondroitin sulfate proteoglycan 4 (CSPG4), a highly immunogenic melanoma tumor antigen, is a potential target for antibody-based immunotherapy. The mechanism by which CSPG4 affects melanoma progressi... |
|
|
A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression.
Biochim. Biophys. Acta 1847 , 1254-66, (2015) Abnormal accumulation of oncometabolite fumarate and succinate is associated with inhibition of mitochondrial function and carcinogenesis. By competing with α-ketoglutarate, oncometabolites also activ... |
|
|
Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells.
Diabetologia 57(8) , 1635-44, (2014) Hypoxic damage complicates islet isolation for transplantation and may contribute to beta cell failure in type 2 diabetes. Polymorphisms in the SLC30A8 gene, encoding the secretory granule zinc transp... |
| Glycine, N-(2-methoxy-1,2-dioxoethyl)-, methyl ester |
| Dimethyloxalylglycine |
| methyl 2-[(2-methoxy-2-oxoethyl)amino]-2-oxoacetate |
| Methyl N-[methoxy(oxo)acetyl]glycinate |
| DMOG |