CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
OJ8480000
-
CHEMICAL NAME :
-
Lobeline
-
CAS REGISTRY NUMBER :
-
90-69-7
-
BEILSTEIN REFERENCE NO. :
-
0091533
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
6
-
MOLECULAR FORMULA :
-
C22-H27-N-O2
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
80 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
AEPPAE Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. (Berlin, Ger.) V.110-253, 1925-66. For publisher information, see NSAPCC. Volume(issue)/page/year: 132,63,1928
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
43500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
STRAAA Strahlentherapie. (Urban & Schwarzenberg, Postfach 202440, D-8000 Munich 2, Fed. Rep. Ger.) V.1- 1912- Volume(issue)/page/year: 127,245,1965
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
AEPPAE Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. (Berlin, Ger.) V.110-253, 1925-66. For publisher information, see NSAPCC. Volume(issue)/page/year: 132,63,1928
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6300 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 103,146,1955
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
35 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 4(1),34,1941
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - flaccid paralysis without anesthesia (usually neuromuscular blockage) Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 31,43,1927
|
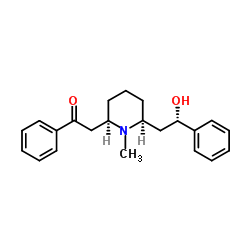
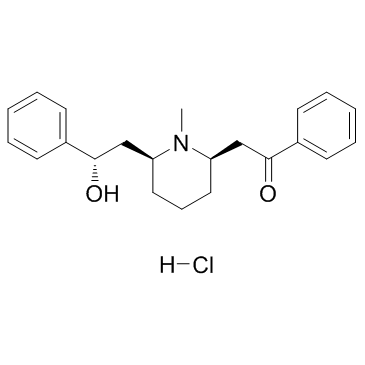 CAS#:134-63-4
CAS#:134-63-4 CAS#:6919-61-5
CAS#:6919-61-5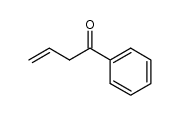 CAS#:6249-80-5
CAS#:6249-80-5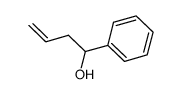 CAS#:80735-94-0
CAS#:80735-94-0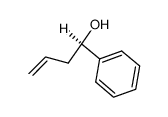 CAS#:85551-57-1
CAS#:85551-57-1 CAS#:423763-79-5
CAS#:423763-79-5![(4S,6R)-4-iodomethyl-6-phenyl[1,3]dioxan-2-one Structure](https://image.chemsrc.com/caspic/134/423763-78-4.png) CAS#:423763-78-4
CAS#:423763-78-4![tert-butyl [(1R)-1-phenylbut-3-enyl] carbonate Structure](https://image.chemsrc.com/caspic/172/423763-77-3.png) CAS#:423763-77-3
CAS#:423763-77-3
