Kallikrein
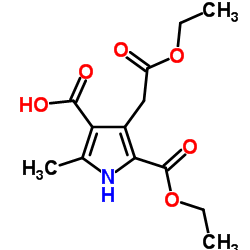
Kallikrein structure
|
Common Name | Kallikrein | ||
|---|---|---|---|---|
| CAS Number | 9001-01-8 | Molecular Weight | 283.277 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 471.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C13H17NO6 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 238.7±28.7 °C | |
Use of KallikreinKallikrein is capable of forming the kallikrenase kalinin system (KKS) in plasma and tissues, producing bradykinin and kalin peptides, respectively[1]. |
| Name | Kallikrein |
|---|---|
| Synonym | More Synonyms |
| Description | Kallikrein is capable of forming the kallikrenase kalinin system (KKS) in plasma and tissues, producing bradykinin and kalin peptides, respectively[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 471.1±45.0 °C at 760 mmHg |
| Molecular Formula | C13H17NO6 |
| Molecular Weight | 283.277 |
| Flash Point | 238.7±28.7 °C |
| Exact Mass | 283.105591 |
| LogP | 2.10 |
| Appearance of Characters | lyophilized powder |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.543 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | B |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NZ2017050 |
|
BMPR2 is required for postimplantation uterine function and pregnancy maintenance.
J. Clin. Invest. 123(6) , 2539-50, (2013) Abnormalities in cell-cell communication and growth factor signaling pathways can lead to defects in maternal-fetal interactions during pregnancy, including immunologic rejection of the fetal/placenta... |
|
|
The structure of human microplasmin in complex with textilinin-1, an aprotinin-like inhibitor from the Australian brown snake.
PLoS ONE 8(1) , e54104, (2013) Textilinin-1 is a Kunitz-type serine protease inhibitor from Australian brown snake venom. Its ability to potently and specifically inhibit human plasmin (K(i) = 0.44 nM) makes it a potential therapeu... |
|
|
Neutron and X-ray crystallographic analysis of Achromobacter protease I at pD 8.0: protonation states and hydration structure in the free-form.
Biochim. Biophys. Acta 1834(8) , 1642-7, (2013) The structure of the free-form of Achromobacter protease I (API) at pD 8.0 was refined by simultaneous use of single crystal X-ray and neutron diffraction data sets to investigate the protonation stat... |
| MFCD00131428 |
| 5-(Ethoxycarbonyl)-4-(2-ethoxy-2-oxoethyl)-2-methyl-1H-pyrrole-3-carboxylic acid |
| 1H-Pyrrole-2,4-dicarboxylic acid, 3-(2-ethoxy-2-oxoethyl)-5-methyl-, 2-ethyl ester |
| kallidinogenase |