Pacritinib
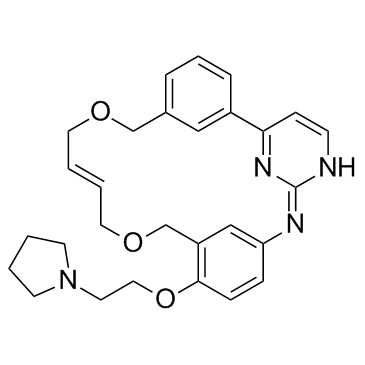
Pacritinib structure
|
Common Name | Pacritinib | ||
|---|---|---|---|---|
| CAS Number | 937272-79-2 | Molecular Weight | 472.579 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 711.4±70.0 °C at 760 mmHg | |
| Molecular Formula | C28H32N4O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 384.0±35.7 °C | |
Use of PacritinibPacritinib is a potent inhibitor of both wild-type JAK2 (IC50=23 nM) and JAK2V617F mutant (IC50=19 nM). Pacritinib also inhibits FLT3 (IC50=22 nM) and its mutant FLT3D835Y (IC50=6 nM). |
| Name | (16E)-11-[2-(1-Pyrrolidinyl)ethoxy]-14,19-dioxa-5,7,27-triazatetracyclo[19.3.1.12,6.18,12]heptacosa-1(25),2(27),3,5,8(26),9,11,16,21,23-decaene |
|---|---|
| Synonym | More Synonyms |
| Description | Pacritinib is a potent inhibitor of both wild-type JAK2 (IC50=23 nM) and JAK2V617F mutant (IC50=19 nM). Pacritinib also inhibits FLT3 (IC50=22 nM) and its mutant FLT3D835Y (IC50=6 nM). |
|---|---|
| Related Catalog | |
| Target |
JAK2V617F:19 nM (IC50) JAK2wt:23 nM (IC50) Tyk2:50 nM (IC50) JAK3:520 nM (IC50) JAK1:1280 nM (IC50) FLT3D835Y:6 nM (IC50) FLT3wt:22 nM (IC50) |
| In Vitro | Relative to JAK2, Pacritinib (SB1518) is two-fold less potent against TYK2 (IC50=50 nM), 23-fold less potent against JAK3 (IC50=520 nM) and 56-fold less potent against JAK1 (IC50=1280 nM). The rest of the evaluated kinases show <30% inhibition when tested against 100 nM Pacritinib at adenosine triphosphate concentrations equivalent to its Michaelis constant (Km). Pacritinib inhibits MV4-11 and MOLM-13 cells (both of which are cell lines derived from human acute myeloid leukemias driven by an FLT3 ITD mutation) with IC50 of 47 and 67 nM, respectively. Pacritinib inhibits Karpas 1106P and Ba/F3-JAK2V617F cells (which are cell lines dependent on JAK2 signaling) with IC50 of 348 and 160 nM, respectively[1]. FLT3-ITD harboring MV4-11 cells are treated for 3 h with different concentrations of Pacritinib (SB1518) and pFLT3, pSTAT5 and pERK1/2 levels are quantified. Pacritinib leads to a dose-dependent decrease of pFLT3, pSTAT5, pERK1/2 and pAkt with IC50 of 80, 40, 33 and 29 nM, respectively. The IC50 on auto-phosphorylation of FLT3-wt in RS4;11 is four-fold higher (IC50=600 nM) compare with FLT3-ITD in MV4-11 and MOLM-13 cells. However, STAT5 inhibition is detected at much lower concentrations of Pacritinib (IC50=8 nM)[2]. |
| In Vivo | For evaluation of efficacy in the Ba/F3-JAK2V617F engraftment model, mice are treated with Pacritinib (SB1518) at doses of 50 or 150 mg/kg p.o. q.d. for 13 days, with drug dosing starting 4 days after cell inoculation. At study termination, the vehicle control mice exhibit splenomegaly and hepatomegaly (~7- and 1.3-fold, respectively), reminiscent of the symptoms found in patients with symptomatic myelofibrosis. SB1518 treatment at 150 mg/kg p.o. q.d. significantly ameliorates all these symptoms, with 60% (±9%) normalization of spleen weight and 92% (±5%) normalization of liver weight and is well tolerated without significant weight loss or any hematological toxicities, including thrombocytopenia and anemia[1]. In rats, Pacritinib (SB1518) shows moderately fast absorption (tmax=4 h), with a peak concentration of 114 ng/mL, AUC of 599 ng•h/mL, and a terminal half-life of ~6 h following a single oral dose of 10 mg/kg. In dogs, Pacritinib (SB1518) is rapidly absorbed (tmax=2.0 h), with a peak concentration of ~12 ng/mL, AUC of 53 ng•h/mL, and a terminal half-life of 3.4 h following a single oral dose of 3 mg/kg[3]. |
| Kinase Assay | All assays are carried out in 384-well white microtiter plates. Compounds (e.g., Pacritinib) are 4-fold serially diluted in 8 steps, starting from 10 µM. The reaction mixture consist of 25 µL assay buffer (50 mM HEPES pH 7.5, 10 mM MgCl2, 5 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 5 mM β-glycerol phosphate). For FLT3 assays, the reaction contain 2.0 µg/mL FLT3 enzyme, 5 µM of poly(Glu,Tyr) substrate and 4 µM of ATP. For JAK1 assays, the reaction contain 2.5 µg/mL of JAK1 enzyme, 10 µM of poly(Glu,Ala,Tyr) substrate and 1.0 µM of ATP. For JAK2 assays, the reaction contain 0.35 µg/mL of JAK2 enzyme, 10 µM of poly (Glu,Ala,Tyr) substrate and 0.15 µM of ATP. For JAK3 assays, the reaction contain 3.5 µg/mL of JAK3 enzyme, 10 µM of poly (Glu,Ala,Tyr) substrate and 6.0 µM of ATP. For TYK2 assays, the reaction contain 2.5 µg/mL of TYK2 enzyme, 10 µM of poly (Glu,Ala,Tyr) substrate and 0.15 µM of ATP. The reaction is incubated at room temperature for 2 h prior to addition of 13 µL PKLight detection reagent. After 10 min incubation luminescent signals are read on a multi-label plate reader[1]. |
| Cell Assay | SET-2 and Karpas 1106P cells, and Ba/F3-JAK2V617F-GFP-Luc cells are used. For proliferation assays in 96-well plates, cells are seeded at 30-50% confluency and are treated the following day with compounds (e.g., Pacritinib) (in triplicate) at concentrations up to 10 μM for 48 h. Cell viability is monitored using the CellTiter-Glo assay. Dose-response curves are plotted to determine IC50 values for the compounds using the XL-fit software[1]. |
| Animal Admin | Mice[1] Female athymic BALB/c nude mice (BALB/cOlaHsd-Foxn1nu) of age 12 weeks are used; and female SCID Beige mice (CB17.Cg-PrkdcscidLystbg/Crl) of age 9-10 weeks are used. For the SET-2 leukemia model, 5×106 tumor cells are injected subcutaneously in the right flank of severe combined immunodeficient beige mice. The cells are resuspended in 50 μL serum-free growth medium, mixed 1:1 with Matrigel and injected in a total volume of 100 μL. Tumor volumes are determined by caliper measurements and drug treatment is initiated after 31 days when tumors have reached a mean volume of 280 mm3 (tumor volume (mm3)=(w2×l)/2). This study is performed using 12 mice per group and animals are killed 3 h post-dose on day 18. Tumor growth inhibition is calculated. For the efficacy studies, mice are treated by oral gavage (10 mL/kg body weight) with doses from 50 to 150 mg/kg SB1518. Rats and Dogs[3] Male Wistar rats (aged 6-8 weeks, weighing 270 to 325 g) and male Beagle dogs (6 to 7 months of age, weighing 9-11 kg) are used in this study. The oral doses for dogs and rats are 3, and 10 mg/kg, respectively. The doses are administered, by gavage, as suspensions (0.5 % methylcellulose and 0.1%tween 80) to rats, and as gelatin capsules to dogs. Following oral dosing, serial blood samples are collected (jugular vein in dogs, and superior vena cava in rats) at different time points (0 to 24 h) in tubes containing K3EDTA as anticoagulant, and centrifuged, the plasma is separated and stored at -70°C until analysis. Plasma samples are processed and analyzed by LC/MS/MS. Pharmacokinetic parameters are estimated by noncompartmental methods using WinNonlin. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 711.4±70.0 °C at 760 mmHg |
| Molecular Formula | C28H32N4O3 |
| Molecular Weight | 472.579 |
| Flash Point | 384.0±35.7 °C |
| Exact Mass | 472.247437 |
| PSA | 68.74000 |
| LogP | 4.23 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.574 |
| Storage condition | -20℃ |
| SB1518 |
| Pacritinib |
| ((2E,16E)-11-[2-(pyrrolidin-1-yl)ethoxy]-14,19-dioxa-5,7,27-triazatetracyclo[19.3.1.12,6.18,12]heptacosa-1(25),2,4,6,8,10,12(26),16,21,23-decaene |
| (16E)-11-[2-(1-Pyrrolidinyl)ethoxy]-14,19-dioxa-5,7,27-triazatetracyclo[19.3.1.1.1]heptacosa-1(25),2(27),3,5,8(26),9,11,16,21,23-decaene |
| 14,19-Dioxa-5,7,27-triazatetracyclo[19.3.1.1.1]heptacosa-1(25),2,4,6(27),8,10,12(26),16,21,23-decaene, 11-[2-(1-pyrrolidinyl)ethoxy]-, (16E)- |