Tetramethylthiuram sulfide
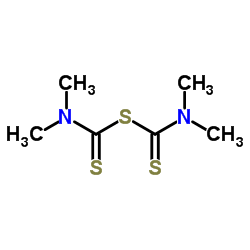
Tetramethylthiuram sulfide structure
|
Common Name | Tetramethylthiuram sulfide | ||
|---|---|---|---|---|
| CAS Number | 97-74-5 | Molecular Weight | 208.368 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 260.9±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H12N2S3 | Melting Point | 107-111 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 111.6±22.6 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of Tetramethylthiuram sulfideTetramethylthiuram monosulfide (TMTM) is an active compound. Tetramethylthiuram monosulfide (TMTM) can be used for the research of rubber and various biochemical studies[1][2]. |
| Name | Bis(Dimethylthiocarbamoyl) Sulfide |
|---|---|
| Synonym | More Synonyms |
| Description | Tetramethylthiuram monosulfide (TMTM) is an active compound. Tetramethylthiuram monosulfide (TMTM) can be used for the research of rubber and various biochemical studies[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 260.9±23.0 °C at 760 mmHg |
| Melting Point | 107-111 °C(lit.) |
| Molecular Formula | C6H12N2S3 |
| Molecular Weight | 208.368 |
| Flash Point | 111.6±22.6 °C |
| Exact Mass | 208.016251 |
| PSA | 95.96000 |
| LogP | 0.87 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.641 |
| InChIKey | REQPQFUJGGOFQL-UHFFFAOYSA-N |
| SMILES | CN(C)C(=S)SC(=S)N(C)C |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317-H411 |
| Precautionary Statements | P273-P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R43;R51/53 |
| Safety Phrases | S24-S26-S37-S61 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | WQ1750000 |
| Packaging Group | III |
| Hazard Class | 9 |
| HS Code | 3812100000 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 3812100000 |
|---|
|
New antimony(III) halide complexes with dithiocarbamate ligands derived from thiuram degradation: The effect of the molecule's close contacts on in vitro cytotoxic activity.
Mater. Sci. Eng. C. Mater. Biol. Appl. 58 , 396-408, (2015) Antimony(III) halide complexes of the formulae {[SbBr(Me2DTC)2]n} (1), {[SbI(Me2DTC)2]n} (2) and {[(Me2DTC)2Sb(μ2-I)Sb(Me2DTC)2](+).I3(-)} (3) (Me2DTC = dimethyldithiocarbomate) were synthesized from ... |
|
|
Occupational allergic contact dermatitis of the ears caused by thiurams in a headset.
Contact Dermatitis 65(4) , 242-3, (2011)
|
|
|
Enzyme inhibition as a possible mechanism of the mutagenicity of dithiocarbamic acid derivatives in Salmonella typhimurium.
Chem. Biol. Interact. 49(3) , 329-40, (1984) In recent years data have accumulated regarding genotoxic properties of dithiocarbamic acid derivatives. The results from the present work indicate that the mutagenicity of these compounds depends on ... |
| Tetramethylthiuram sulfide |
| Trithiodicarbonic diamide, N,N,N',N'-tetramethyl- |
| Sulfide, bis[(dimethylamino)thioxomethyl] |
| EINECS 202-605-7 |
| N,N,N',N'-Tetramethyldicarbonotrithioic diamide |
| Bis(dimethylthiocarbamyl) sulfide |
| MFCD00014870 |
| Bis(dimethylthiocarbamyl) monosulfide |
| TMTM |
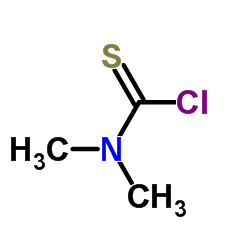 CAS#:16420-13-6
CAS#:16420-13-6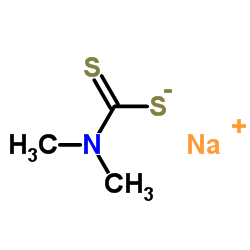 CAS#:128-04-1
CAS#:128-04-1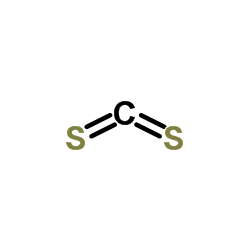 CAS#:75-15-0
CAS#:75-15-0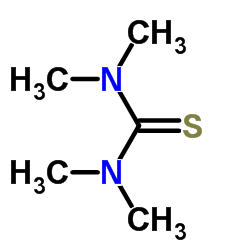 CAS#:2782-91-4
CAS#:2782-91-4 CAS#:124-40-3
CAS#:124-40-3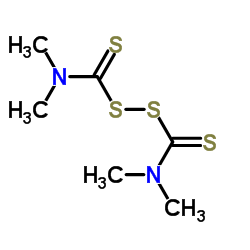 CAS#:137-26-8
CAS#:137-26-8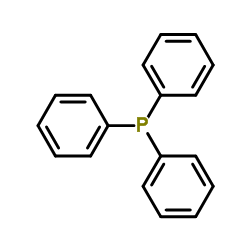 CAS#:603-35-0
CAS#:603-35-0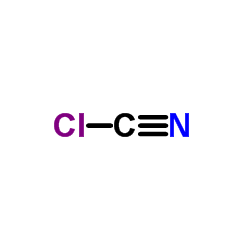 CAS#:506-77-4
CAS#:506-77-4 CAS#:143-33-9
CAS#:143-33-9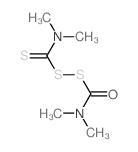 CAS#:1115-06-6
CAS#:1115-06-6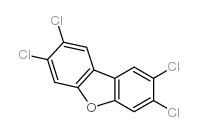 CAS#:51207-31-9
CAS#:51207-31-9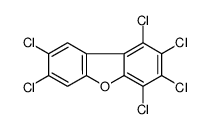 CAS#:70648-26-9
CAS#:70648-26-9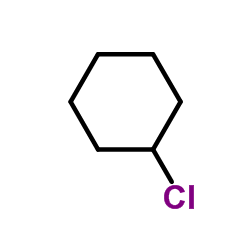 CAS#:542-18-7
CAS#:542-18-7 CAS#:4837-38-1
CAS#:4837-38-1 CAS#:102-08-9
CAS#:102-08-9 CAS#:27849-33-8
CAS#:27849-33-8
