(-)-Epigallocatechin(EGC)

(-)-Epigallocatechin(EGC) structure
|
Common Name | (-)-Epigallocatechin(EGC) | ||
|---|---|---|---|---|
| CAS Number | 970-74-1 | Molecular Weight | 306.267 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 685.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H14O7 | Melting Point | 208-210°C | |
| MSDS | Chinese USA | Flash Point | 368.5±31.5 °C | |
Use of (-)-Epigallocatechin(EGC)(-)-Epigallocatechin is the most abundant flavonoid in green tea, can bind to unfolded native polypeptides and prevent conversion to amyloid fibrils.IC50 value:Target: in vitro: EGCG is a potent inhibitor of amyloidogenic cystatin I66Q amyloid fibril formation in vitro. Computational analysis suggests that EGCG prevents amyloidogenic cystatin fibril formation by stabilizing the molecule in its native-like state as opposed to redirecting aggregation to disordered, amorphous aggregates [1]. Combined curcumin and EGCG treatment reduced the cancer stem-like Cluster of differentiation 44 (CD44)-positive cell population. Western blot and immunoprecipitation analyses revealed that curcumin and EGCG specifically inhibited STAT3 phosphorylation and STAT3-NFkB interaction was retained [2]. EGCG exhibited a MIC and MBC of 5μg/mL and 20μg/mL respectively and effectively eradicated E. faecalis biofilms. EGCG induced the formation of hydroxyl radicals in E. faecalis. The addition of DIP protected E. faecalis against EGCG-mediated antibacterial effects. At sub-MIC, EGCG induced significant down-regulation of E. faecalis virulence genes [3]. |
| Name | (-)-epigallocatechin |
|---|---|
| Synonym | More Synonyms |
| Description | (-)-Epigallocatechin is the most abundant flavonoid in green tea, can bind to unfolded native polypeptides and prevent conversion to amyloid fibrils.IC50 value:Target: in vitro: EGCG is a potent inhibitor of amyloidogenic cystatin I66Q amyloid fibril formation in vitro. Computational analysis suggests that EGCG prevents amyloidogenic cystatin fibril formation by stabilizing the molecule in its native-like state as opposed to redirecting aggregation to disordered, amorphous aggregates [1]. Combined curcumin and EGCG treatment reduced the cancer stem-like Cluster of differentiation 44 (CD44)-positive cell population. Western blot and immunoprecipitation analyses revealed that curcumin and EGCG specifically inhibited STAT3 phosphorylation and STAT3-NFkB interaction was retained [2]. EGCG exhibited a MIC and MBC of 5μg/mL and 20μg/mL respectively and effectively eradicated E. faecalis biofilms. EGCG induced the formation of hydroxyl radicals in E. faecalis. The addition of DIP protected E. faecalis against EGCG-mediated antibacterial effects. At sub-MIC, EGCG induced significant down-regulation of E. faecalis virulence genes [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 685.6±55.0 °C at 760 mmHg |
| Melting Point | 208-210°C |
| Molecular Formula | C15H14O7 |
| Molecular Weight | 306.267 |
| Flash Point | 368.5±31.5 °C |
| Exact Mass | 306.073944 |
| PSA | 130.61000 |
| LogP | -0.10 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.776 |
| InChIKey | XMOCLSLCDHWDHP-IUODEOHRSA-N |
| SMILES | Oc1cc(O)c2c(c1)OC(c1cc(O)c(O)c(O)c1)C(O)C2 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | KB5100000 |
| HS Code | 2932999099 |
| Precursor 4 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effects of malted and non-malted whole-grain wheat on metabolic and inflammatory biomarkers in overweight/obese adults: a randomised crossover pilot study.
Food Chem. 194 , 495-502, (2015) Metabolic dysfunction in obesity may be attenuated by whole-grain intake, which has been attributed to synergistic actions of bioactive compounds. Germination/malting may increase grain bioactives, in... |
|
|
A colourimetric sensor for the simultaneous determination of oxidative status and antioxidant activity on the same membrane: N,N-dimethyl-p-phenylene diamine (DMPD) on Nafion.
Anal. Chim. Acta 865 , 60-70, (2015) A colourimetric sensor capable of simultaneously measuring oxidative status (OS) in terms of the hazard produced by reactive oxygen species (ROS) and antioxidant activity (AOA) in regard to ROS-scaven... |
|
|
Identification and quantification of phytochemicals in nutraceutical products from green tea by UHPLC-Orbitrap-MS.
Food Chem. 173 , 607-18, (2014) A method has been developed and validated for the simultaneous detection and quantification of phytochemicals in nutraceutical products obtained from green tea. For that purpose, ultra-high performanc... |
| EGC |
| MFCD00075939 |
| Galloepicatechin |
| epigallocatechol |
| Sunphenon EGC |
| (-)-Epigallo catechin |
| (-)-Epigallocatechin |
| (−)-Epigallocatechin |
| (-)-Epigallocatechin (EGC) |
| (2R,3R)-2-(3,4,5-Trihydroxyphenyl)-3,5,7-chromanetriol |
| Teacatechin II |
| EPIGALLOCATECHIN |
| (2R,3R)-2-(3,4,5-Trihydroxyphenyl)chromane-3,5,7-triol |
| Greenteacatechins |
| (-)-EGC:2H-1-Benzopyran-3,5,7-triol,3,4-dihydro-2-(3,4,5-trihydroxyphenyl)-, (2R-cis)-, |
| 2H-1-Benzopyran-3,5,7-triol, 3,4-dihydro-2-(3,4,5-trihydroxyphenyl)-, (2R,3R)- |
| epi |
![(-)-(2R,3R)-cis-5,7-bis(benzyloxy)-2-[3,4,5-tris(benzyloxy)phenyl]chroman-3-ol Structure](https://image.chemsrc.com/caspic/078/332386-74-0.png) CAS#:332386-74-0
CAS#:332386-74-0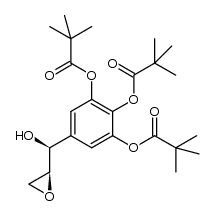 CAS#:1449477-27-3
CAS#:1449477-27-3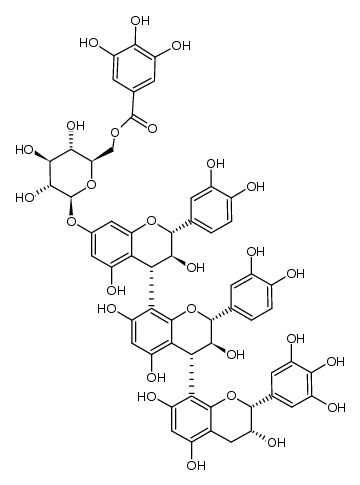 CAS#:1333874-50-2
CAS#:1333874-50-2 CAS#:108-98-5
CAS#:108-98-5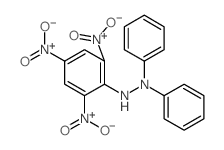 CAS#:1707-75-1
CAS#:1707-75-1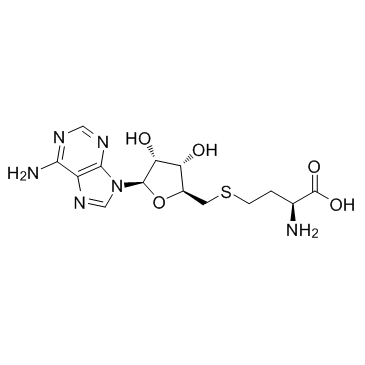 CAS#:979-92-0
CAS#:979-92-0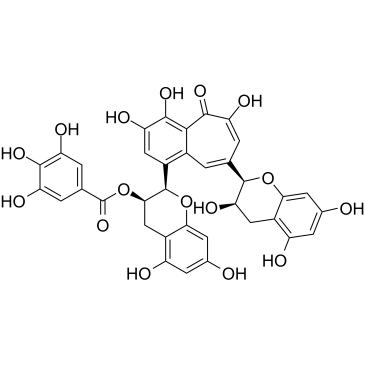 CAS#:28543-07-9
CAS#:28543-07-9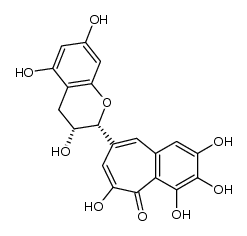 CAS#:102208-16-2
CAS#:102208-16-2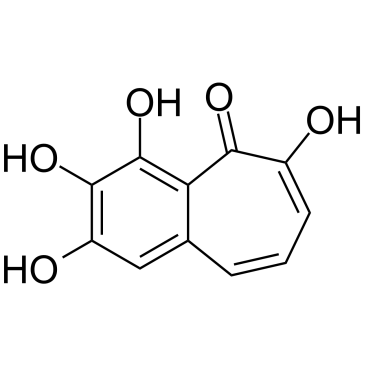 CAS#:569-77-7
CAS#:569-77-7 CAS#:31129-95-0
CAS#:31129-95-0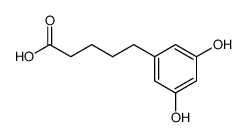 CAS#:74356-41-5
CAS#:74356-41-5 CAS#:1204316-08-4
CAS#:1204316-08-4 CAS#:4670-05-7
CAS#:4670-05-7 CAS#:302776-47-2
CAS#:302776-47-2
