3-Aminoacetophenone
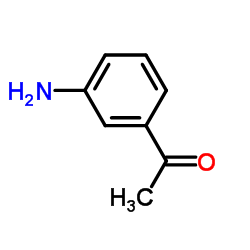
3-Aminoacetophenone structure
|
Common Name | 3-Aminoacetophenone | ||
|---|---|---|---|---|
| CAS Number | 99-03-6 | Molecular Weight | 135.16 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 290.3±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H9NO | Melting Point | 94-98 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 129.4±19.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3-Aminoacetophenone1-(3-Aminophenyl)ethanone is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 3-Aminoacetophenone |
|---|---|
| Synonym | More Synonyms |
| Description | 1-(3-Aminophenyl)ethanone is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 290.3±13.0 °C at 760 mmHg |
| Melting Point | 94-98 °C(lit.) |
| Molecular Formula | C8H9NO |
| Molecular Weight | 135.16 |
| Flash Point | 129.4±19.8 °C |
| Exact Mass | 135.068420 |
| PSA | 43.09000 |
| LogP | 0.69 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.571 |
| InChIKey | CKQHAYFOPRIUOM-UHFFFAOYSA-N |
| SMILES | CC(=O)c1cccc(N)c1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AM5800000 |
| HS Code | 29223900 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922399090 |
|---|---|
| Summary | 2922399090 other amino-aldehydes, amino-ketones and amino-quinones, other than those containing more than one kind of oxygen function; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Enantioselective synthesis of pactamycin, a complex antitumor antibiotic.
Science 340(6129) , 180-2, (2013) Medicinal application of many complex natural products is precluded by the impracticality of their chemical synthesis. Pactamycin, the most structurally intricate aminocyclopentitol antibiotic, displa... |
|
|
Synthesis of sulfonyl curcumin mimics exerting a vasodilatation effect on the basilar artery of rabbits.
Bioorg. Med. Chem. Lett. 19(5) , 1481-3, (2009) In order to discover novel small vasodilatory molecules for potential use in the treatment of vascular disease, we tested the vasodilatation effect of two types of synthetic curcumin mimics, amide typ... |
|
|
Symmetric building blocks and combinatorial functional group transformation as versatile strategies in combinatorial chemistry.
Biotechnol. Bioeng. 71(2) , 94-103, (2000) A combination of symmetric building blocks and combinatorial functional group transformation for synthesis of pyrimidines was investigated. The purpose of the study was to maximize the return on inves... |
| Ethanone, 1-(3-aminophenyl)- |
| EINECS 202-722-3 |
| 3-acetylaniline |
| m-Acetylaniline |
| 3-Amino acetophenone |
| 1-(3-Aminophenyl)ethanone |
| M-AMINOACETOPHENONE |
| m-Aminoacetylbenzene |
| MFCD00007796 |
 CAS#:121-89-1
CAS#:121-89-1 CAS#:2142-63-4
CAS#:2142-63-4 CAS#:54060-30-9
CAS#:54060-30-9 CAS#:99-02-5
CAS#:99-02-5 CAS#:70334-60-0
CAS#:70334-60-0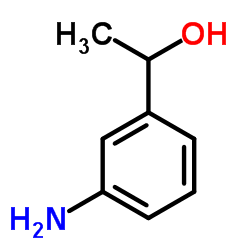 CAS#:2454-37-7
CAS#:2454-37-7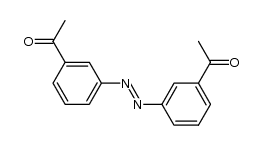 CAS#:151224-49-6
CAS#:151224-49-6 CAS#:98-86-2
CAS#:98-86-2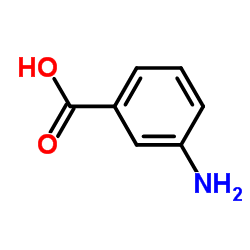 CAS#:99-05-8
CAS#:99-05-8 CAS#:3125-71-1
CAS#:3125-71-1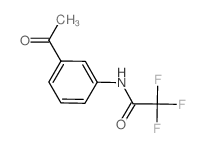 CAS#:56915-87-8
CAS#:56915-87-8 CAS#:455-36-7
CAS#:455-36-7 CAS#:52780-14-0
CAS#:52780-14-0 CAS#:55-21-0
CAS#:55-21-0 CAS#:1166988-10-8
CAS#:1166988-10-8 CAS#:149914-98-7
CAS#:149914-98-7 CAS#:37148-51-9
CAS#:37148-51-9 CAS#:51226-14-3
CAS#:51226-14-3 CAS#:194783-82-9
CAS#:194783-82-9
